Korean J Physiol Pharmacol.
2017 Sep;21(5):465-474. 10.4196/kjpp.2017.21.5.465.
Resveratrol raises in vitro anticancer effects of paclitaxel in NSCLC cell line A549 through COX-2 expression
- Affiliations
-
- 1School of Medicine, Shandong University, Jinan 250000, Shandong, China.
- 2Department of Thoracic Surgery, Taian City Central Hospital, Taian 271000, Shandong, China. wangzhousd@qq.com
- 3Department of Gynaecology and Obstetrics, Taian City Central Hospital, Taian 271000, Shandong, China.
- 4Taian Vocational College of Nursing, Taian 271000, Shandong, China.
- 5Department of Thoracic Surgery, Shandong Provincial Hospital affiliated to Shandong University, Jinan 250000, Shandong, China. 3454332590@qq.com
- KMID: 2388692
- DOI: http://doi.org/10.4196/kjpp.2017.21.5.465
Abstract
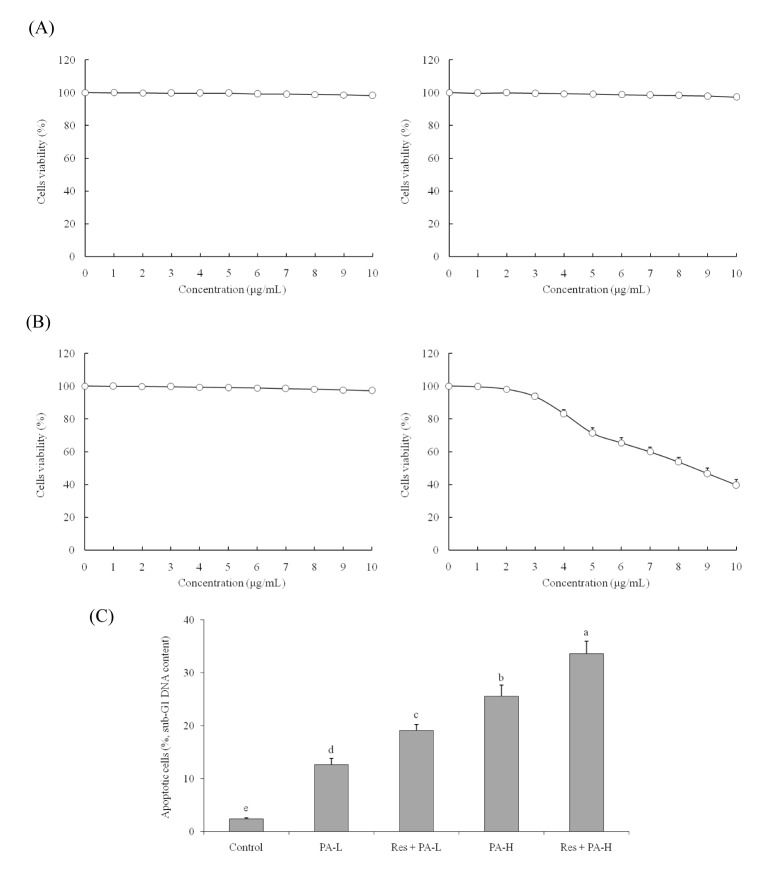
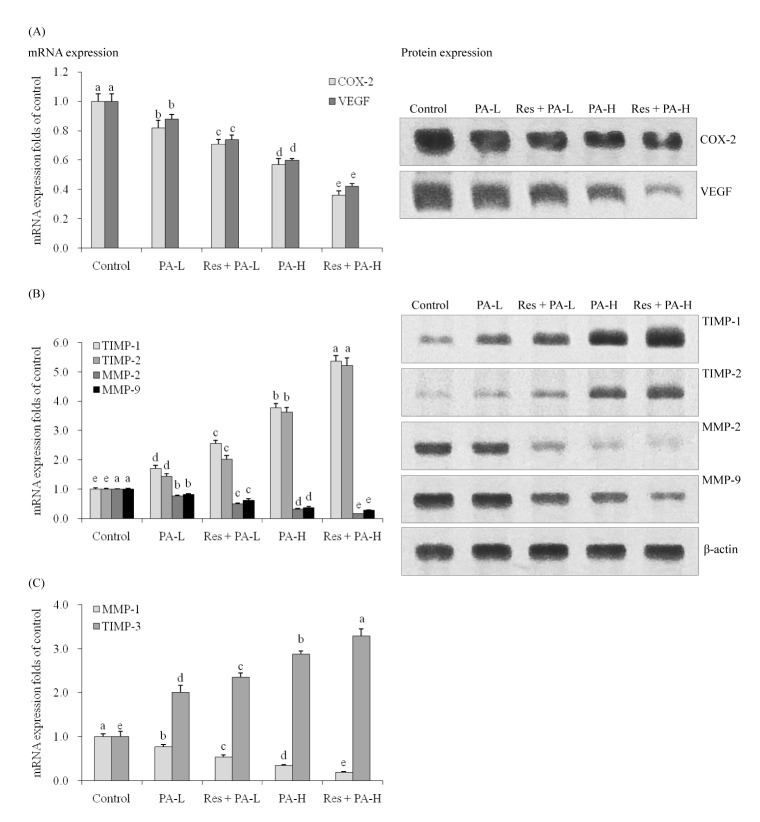
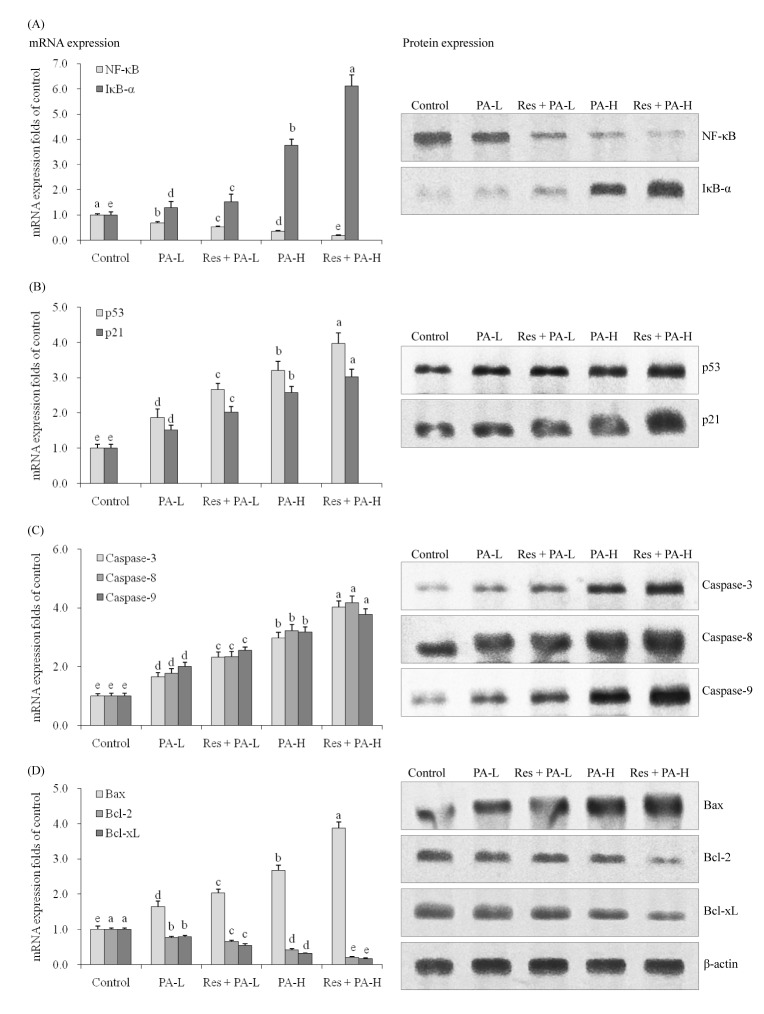
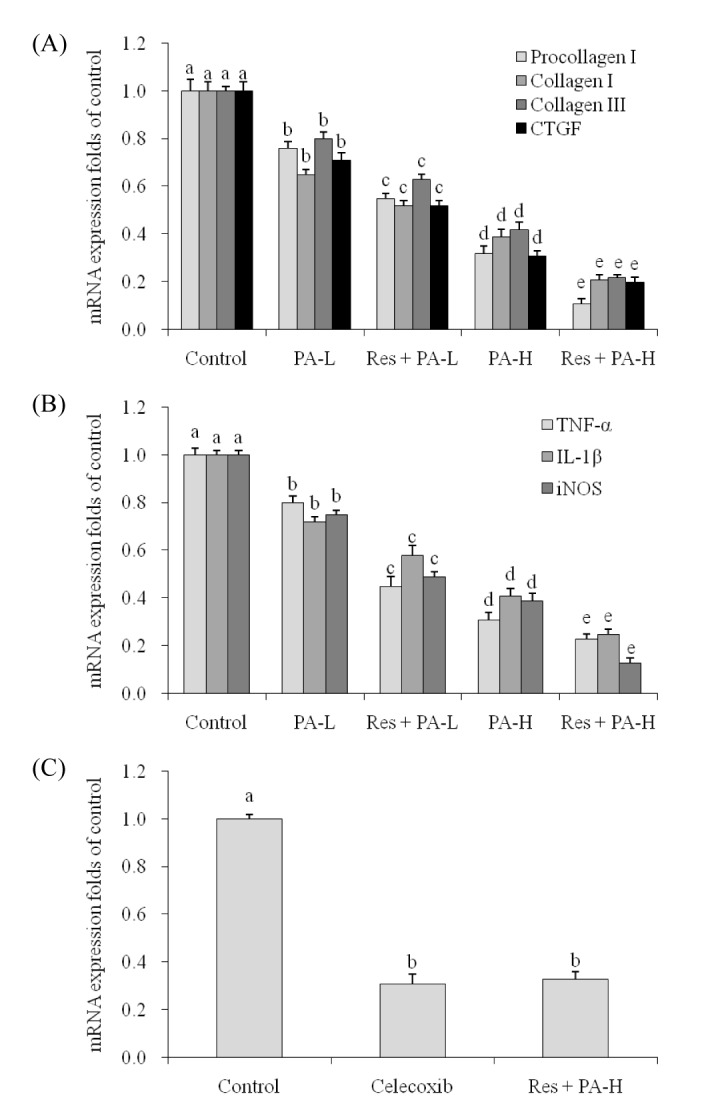
- The aim of this study was to determine the raising anticancer effects of resveratrol (Res) on paclitaxel (PA) in non-small cell lung cancer (NSCLC) cell line A549. The 10 µg/ml of Res had no effect on human fetal lung fibroblast MRC-5 cells or on A549 cancer cells and the 5 or 10 µg/ml of PA also had no effect on MRC-5 normal cells. PA-L (5 µg/ml) and PA-H (10 µg/ml) had the growth inhibitory effects in NSCLC cell line A549, and Res increased these growth inhibitory effects. By flow cytometry experiment, after Res (5 µg/ml)+PA-H (10 µg/ml) treatment, the A549 cells showed the most apoptosic cells compared to other group treatments, and after additional treatment with Res, the apoptosic cells of both two PA concentrations were raised. Res+PA could reduce the mRNA and protein expressions of COX-2, and Res+PA could reduce the COX-2 related genes of VEGF, MMP-1, MMP-2, MMP-9, NF-κB, Bcl-2, Bcl-xL, procollagen I, collagen I, collagen III and CTGF, TNF-α, IL-1β, iNOS and raise the TIMP-1, TIMP-2, TIMP-3, IκB-α, p53, p21, caspase-3, caspase-8, caspase-9, Bax genes compared to the control cells and the PA treated cells. From these results, it can be suggested that Res could raise the anticancer effects of PA in A549 cells, thus Res might be used as a good sensitizing agent for PA.
Keyword
MeSH Terms
-
Carcinoma, Non-Small-Cell Lung
Caspase 3
Caspase 8
Caspase 9
Cell Line*
Collagen
Fibroblasts
Flow Cytometry
Humans
In Vitro Techniques*
Lung
Paclitaxel*
Procollagen
RNA, Messenger
Tissue Inhibitor of Metalloproteinase-1
Tissue Inhibitor of Metalloproteinase-2
Tissue Inhibitor of Metalloproteinase-3
Vascular Endothelial Growth Factor A
Caspase 3
Caspase 8
Caspase 9
Collagen
Paclitaxel
Procollagen
RNA, Messenger
Tissue Inhibitor of Metalloproteinase-1
Tissue Inhibitor of Metalloproteinase-2
Tissue Inhibitor of Metalloproteinase-3
Vascular Endothelial Growth Factor A
Figure
Reference
-
1. Davidson M, Smyth EC, Cunningham D. Clinical role of ramucirumab alone or in combination with paclitaxel for gastric and gastro-esophageal junction adenocarcinoma. Onco Targets Ther. 2016; 9:4539–4548. PMID: 27524910.2. Yu B, Tan L, Zheng R, Tan H, Zheng L. Targeted delivery and controlled release of Paclitaxel for the treatment of lung cancer using single-walled carbon nanotubes. Mater Sci Eng C Mater Biol Appl. 2016; 68:579–584. PMID: 27524057.
Article3. Cunha KS, Reguly ML, Graf U, de Andrade HH. Taxanes: the genetic toxicity of paclitaxel and docetaxel in somatic cells of Drosophila melanogaster. Mutagenesis. 2001; 16:79–84. PMID: 11139602.
Article4. Shi WQ. Historical story on natural medicinal chemistry of Taxol. Chinese Tradi Herbal Drug. 2011; 42:1878–1884.5. Liu D, Kang H, Sun C, Xiao Y, Zhao K. Paclitaxel induced apoptotic genes and pathways alterations: a review. Sheng Wu Gong Cheng Xue Bao. 2013; 29:153–160. PMID: 23697160.6. Varoni EM, Lo Faro AF, Sharifi-Rad J, Iriti M. Anticancer molecular mechanisms of resveratrol. Front Nutr. 2016; 3:8. PMID: 27148534.
Article7. Zulueta A, Caretti A, Signorelli P, Ghidoni R. Resveratrol: a potential challenger against gastric cancer. World J Gastroenterol. 2015; 21:10636–10643. PMID: 26457023.
Article8. Jang M, Pezzuto JM. Effects of resveratrol on 12-O-tetradecanoylphorbol-13-acetate-induced oxidative events and gene expression in mouse skin. Cancer Lett. 1998; 134:81–89. PMID: 10381133.
Article9. Bai HN, Wang ZY, Liu RH, Zhao HT, Zhang H. Synergistic ABTS radical scavenging activity of resveratrol with Auricularia auricular polysaccharides. Mod Food Sci Technol. 2014; 30:64–68.10. Xing XM, Wang Y, Du LQ, Xu C, Lin KL, Fan SJ, Liu Q. Study on radiosensitization effect and mechanism of resveratrol on lung A549 cells. J Radiat Res Radiat Process. 2014; 32:060203.11. Huang H, Al-Shabrawey M, Wang MH. Cyclooxygenase- and cytochrome P450-derived eicosanoids in stroke. Prostaglandins Other Lipid Mediat. 2016; 122:45–53. PMID: 26747234.
Article12. Echizen K, Hirose O, Maeda Y, Oshima M. Inflammation in gastric cancer: Interplay of the COX-2/prostaglandin E2 and Toll-like receptor/MyD88 pathways. Cancer Sci. 2016; 107:391–397. PMID: 27079437.13. Zhao X, Kim SY, Park KY. Bamboo salt has in vitro anticancer activity in HCT-116 cells and exerts anti-metastatic effects in vivo. J Med Food. 2013; 16:9–19. PMID: 23256441.14. Li L, Gao M, Song B, Zhang H, Wang Y. Effects of RECQ1 helicase silencing on non-small cell lung cancer cells. Biomed Pharmacother. 2016; 83:1227–1232. PMID: 27565844.
Article15. Feng Y, Renshaw S, Martin P. Live imaging of tumor initiation in zebrafish larvae reveals a trophic role for leukocyte-derived PGE2. Curr Biol. 2012; 22:1253–1259. PMID: 22658594.16. Shiota G, Okubo M, Noumi T, Noguchi N, Oyama K, Takano Y, Yashima K, Kishimoto Y, Kawasaki H. Cyclooxygenase-2 expression in hepatocellular carcinoma. Hepatogastroenterology. 1999; 46:407–412. PMID: 10228831.17. Xu B, Wang Y, Yang J, Zhang Z, Zhang Y, Du H. Celecoxib induces apoptosis but up-regulates VEGF via endoplasmic reticulum stress in human colorectal cancer in vitro and in vivo. Cancer Chemother Pharmacol. 2016; 77:797–806. PMID: 26931344.
Article18. Li N, Zheng D, Xue J, Guo W, Shi J, Sun J, Lu C, Zheng W, Wu M, Cheng S. Cidan inhibits liver cancer cell growth by reducing COX-2 and VEGF expression and cell cycle arrest. Exp Ther Med. 2015; 9:1709–1718. PMID: 26136881.
Article19. Kurihara Y, Hatori M, Ando Y, Ito D, Toyoshima T, Tanaka M, Shintani S. Inhibition of cyclooxygenase-2 suppresses the invasiveness of oral squamous cell carcinoma cell lines via down-regulation of matrix metalloproteinase-2 production and activation. Clin Exp Metastasis. 2009; 26:425–432. PMID: 19241124.
Article20. Park SY, Kim YH, Kim Y, Lee SJ. Aromatic-turmerone attenuates invasion and expression of MMP-9 and COX-2 through inhibition of NF-κB activation in TPA-induced breast cancer cells. J Cell Biochem. 2012; 113:3653–3662. PMID: 22740037.
Article21. Abiru S, Nakao K, Ichikawa T, Migita K, Shigeno M, Sakamoto M, Ishikawa H, Hamasaki K, Nakata K, Eguchi K. Aspirin and NS-398 inhibit hepatocyte growth factor-induced invasiveness of human hepatoma cells. Hepatology. 2002; 35:1117–1124. PMID: 11981761.
Article22. Kim YI, Park SW, Yoon YK, Lee KW, Lee JH, Woo HJ, Kim Y. Orostachys japonicus inhibits the expression of MMP-2 and MMP-9 mRNA and modulates the expression of iNOS and COX-2 genes in human PMA-differentiated THP-1 cells via inhibition of NF-κB and MAPK activation. Mol Med Rep. 2015; 12:657–662. PMID: 25760396.
Article23. Hilska M, Roberts PJ, Collan YU, Laine VJ, Kössi J, Hirsimäki P, Rahkonen O, Laato M. Prognostic significance of matrix metalloproteinases-1, -2, -7 and -13 and tissue inhibitors of metalloproteinases-1, -2, -3 and -4 in colorectal cancer. Int J Cancer. 2007; 121:714–723. PMID: 17455256.
Article24. Gu P, Xing X, Tänzer M, Röcken C, Weichert W, Ivanauskas A, Pross M, Peitz U, Malfertheiner P, Schmid RM, Ebert MP. Frequent loss of TIMP-3 expression in progression of esophageal and gastric adenocarcinomas. Neoplasia. 2008; 10:563–572. PMID: 18516293.
Article25. Wang Y, Huang G, Mo B, Wang C. Artesunate modulates expressionof matrix metalloproteinases and their inhibitors as well as collagen-IV to attenuate pulmonary fibrosis in rats. Genet Mol Res. 2016; 15:DOI: 10.4238/gmr.15027530.
Article26. Yodkeeree S, Pompimon W, Limtrakul P. Crebanine, an aporphine alkaloid, sensitizes TNF-α-induced apoptosis and suppressed invasion of human lung adenocarcinoma cells A549 by blocking NF-κB-regulated gene products. Tumour Biol. 2014; 35:8615–8624. PMID: 24867094.
Article27. Gomes TS, Noguti J, Forones NM, Lima FO, Dobo C, Fernandes Junior JA, Oshima CT, Ribeiro DA. Correlation analysis of c-myc, p21(WAF/CIP1), p53, C-erbB-2 and COX-2 proteins in esophageal squamous cell carcinoma. Pathol Res Pract. 2013; 209:6–9. PMID: 23177619.
Article28. Kim BM, Won J, Maeng KA, Han YS, Yun YS, Hong SH. Nimesulide, a selective COX-2 inhibitor, acts synergistically with ionizing radiation against A549 human lung cancer cells through the activation of caspase-8 and caspase-3. Int J Oncol. 2009; 34:1467–1473. PMID: 19360361.
Article29. Singh M, Singh N. Molecular mechanism of curcumin induced cytotoxicity in human cervical carcinoma cells. Mol Cell Biochem. 2009; 325:107–119. PMID: 19191010.
Article30. Liu Y, Shi QF, Qi M, Tashiro S, Onodera S, Ikejima T. Interruption of hepatocyte growth factor signaling augmented oridonin-induced death in human non-small cell lung cancer A549 cells via c-met-nuclear factor-κB-cyclooxygenase-2 and c-Met-Bcl-2-caspase-3 pathways. Biol Pharm Bull. 2012; 35:1150–1158. PMID: 22791165.31. Gallouet AS, Travert M, Bresson-Bepoldin L, Guilloton F, Pangault C, Caulet-Maugendre S, Lamy T, Tarte K, Guillaudeux T. COX-2-independent effects of celecoxib sensitize lymphoma B cells to TRAIL-mediated apoptosis. Clin Cancer Res. 2014; 20:2663–2673. PMID: 24637636.
Article32. Chien CC, Ko CH, Shen SC, Yang LY, Chen YC. The role of COX-2/PGE2 in gossypol-induced apoptosis of colorectal carcinoma cells. J Cell Physiol. 2012; 227:3128–3137. PMID: 22170686.
Article33. Chandramohan Reddy T, Bharat Reddy D, Aparna A, Arunasree KM, Gupta G, Achari C, Reddy GV, Lakshmipathi V, Subramanyam A, Reddanna P. Anti-leukemic effects of gallic acid on human leukemia K562 cells: downregulation of COX-2, inhibition of BCR/ABL kinase and NF-κB inactivation. Toxicol In Vitro. 2012; 26:396–405. PMID: 22245431.
Article34. Bracke ME, Parmar VS, Depass AL, Stevens CV, Vanhoecke BW, Mareel MM. Chick heart invasion assay. Methods Mol Biol. 2014; 1070:93–106. PMID: 24092434.
Article35. Gilles C, Polette M, Seiki M, Birembaut P, Thompson EW. Implication of collagen type I-induced membrane-type 1-matrix metalloproteinase expression and matrix metalloproteinase-2 activation in the metastatic progression of breast carcinoma. Lab Invest. 1997; 76:651–660. PMID: 9166284.36. Shintani Y, Maeda M, Chaika N, Johnson KR, Wheelock MJ. Collagen I promotes epithelial-to-mesenchymal transition in lung cancer cells via transforming growth factor-beta signaling. Am J Respir Cell Mol Biol. 2008; 38:95–104. PMID: 17673689.37. Song JL, Qian Y, Li GJ, Zhao X. Anti-inflammatory effects of kudingcha methanol extract (Ilex kudingcha C.J. Tseng) in dextran sulfate sodium-induced ulcerative colitis. Mol Med Rep. 2013; 8:1256–1262. PMID: 23969782.
Article38. Dionne S, D'Agata ID, Hiscott J, Vanounou T, Seidman EG. Colonic explant production of IL-1and its receptor antagonist is imbalanced in inflammatory bowel disease (IBD). Clin Exp Immunol. 1998; 112:435–442. PMID: 9649212.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- alpha-Tocopheryl succinate potentiates the paclitaxel-induced apoptosis through enforced caspase 8 activation in human H460 lung cancer cells
- Relation between Cyclooxygenase-2 and Polo-like Kinase-1 in Non-Small Cell Lung Cancer
- Cytotoxicity of COX-2 Inhibitor (Nimesulide) in Non-small Cell Lung Cancer Cell Line
- MicroRNA-373 Inhibits Cell Proliferation and Invasion via Targeting BRF2 in Human Non-small Cell Lung Cancer A549 Cell Line
- Expression of COX-2 and IDO by Uteroglobin Transduction in NSCLC Cell Lines