Tuberc Respir Dis.
2009 Oct;67(4):303-310.
Relation between Cyclooxygenase-2 and Polo-like Kinase-1 in Non-Small Cell Lung Cancer
- Affiliations
-
- 1Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine and Lung Institute, Seoul National University, College of Medicine, Seoul, Korea. scyang@snu.ac.kr
Abstract
- BACKGROUND
Elevated expression of cyclooxygenase-2 (COX-2) and Polo-like kinase-1 (PLK-1) is observed in a wide variety of cancers. Augmented expression of COX-2 and enhanced production of prostaglandin E2 (PGE2) are associated with increased tumor cell survival and malignancy; COX-2 has been implicated in the control of human non-small cell lung carcinoma (NSCLC) cell growth. PLK-1 siRNA induced the cell death of lung cancer cells and the systemic administration of PLK-1 siRNA/atelocollagen complex inhibited the growth of lung cancer in a liver metastatic murine model. COX-2 and PLK-1 are involved in proliferation and in cell cycle regulation, and there is a significant correlation between their interaction in prostate carcinoma.
METHODS
In this study, we investigated the pattern of COX-2 and PLK-1 expression in NSCLC, after treatment with IL-1beta, COX-2 inhibitor and PLK-1 siRNA.
RESULTS
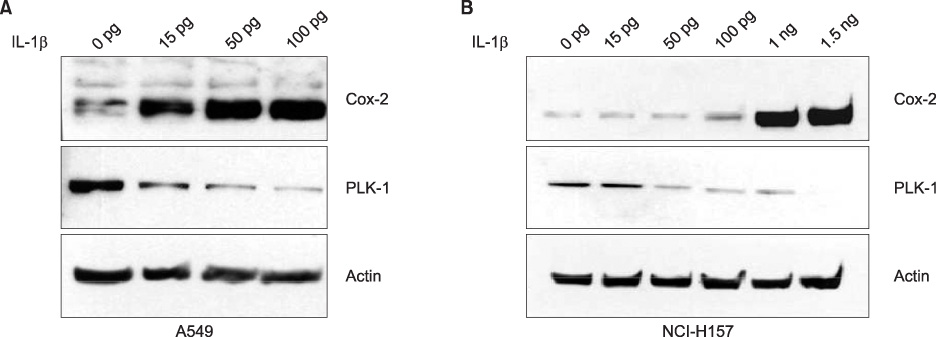
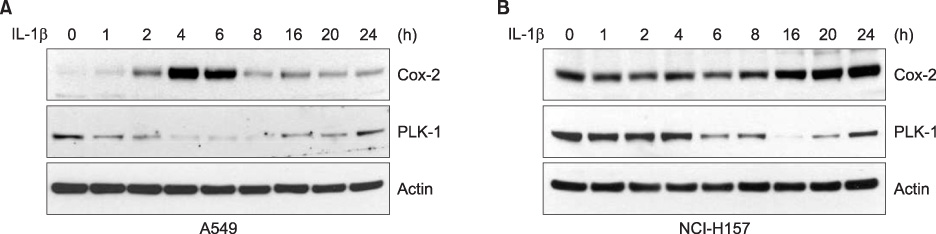
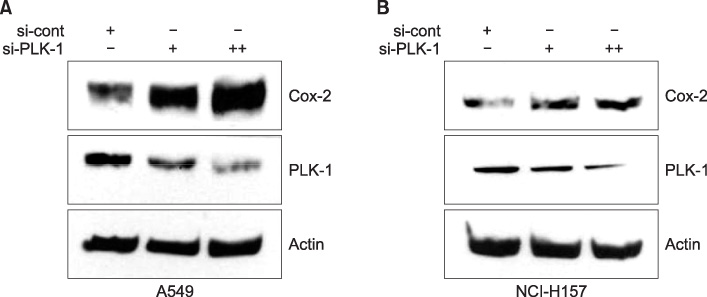
Expression of PLK-1 was decreased in A549 COX-2 sense cells, and was increased in A549 COX-2 anti-sense cells. Knock out of PLK-1 expression by PLK-1 siRNA augmented COX-2 expression in A549 and NCl-H157 cells. When A549 and NCI-H157 cells were treated with COX-2 inhibitor on a dose-dependent basis, PLK-1 and COX-2 were reduced. However, when the expression of COX-2 was induced by IL-1beta, the production of PLK-1 decreased.
CONCLUSION
These results demonstrate that COX-2 and PLK-1 are regulated and inhibited by each other in NSCLC, and suggest that these proteins have a reverse relationship in NSCLC.
Keyword
MeSH Terms
-
Carcinoma, Non-Small-Cell Lung
Cell Cycle
Cell Cycle Proteins
Cell Death
Cell Survival
Cyclooxygenase 2
Dinoprostone
Humans
Liver
Lung
Lung Neoplasms
Prostate
Protein-Serine-Threonine Kinases
Proteins
Proto-Oncogene Proteins
RNA, Small Interfering
Cell Cycle Proteins
Cyclooxygenase 2
Dinoprostone
Protein-Serine-Threonine Kinases
Proteins
Proto-Oncogene Proteins
RNA, Small Interfering
Figure
Reference
-
1. Chen CC, Sun YT, Chen JJ, Chiu KT. TNF-alpha-induced cyclooxygenase-2 expression in human lung epithelial cells: involvement of the phospholipase C-gamma 2, protein kinase C-alpha, tyrosine kinase, NF-kappa B-inducing kinase, and I-kappa B kinase 1/2 pathway. J Immunol. 2000. 165:2719–2728.2. Mitchell JA, Akarasereenont P, Thiemermann C, Flower RJ, Vane JR. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc Natl Acad Sci U S A. 1993. 90:11693–11697.3. Maier JA, Hla T, Maciag T. Cyclooxygenase is an immediate-early gene induced by interleukin-1 in human endothelial cells. J Biol Chem. 1990. 265:10805–10808.4. Lee SH, Soyoola E, Chanmugam P, Hart S, Sun W, Zhong H, et al. Selective expression of mitogen-inducible cyclooxygenase in macrophages stimulated with lipopolysaccharide. J Biol Chem. 1992. 267:25934–25938.5. Hasturk S, Kemp B, Kalapurakal SK, Kurie JM, Hong WK, Lee JS. Expression of cyclooxygenase-1 and cyclooxygenase-2 in bronchial epithelium and nonsmall cell lung carcinoma. Cancer. 2002. 94:1023–1031.6. Yoon JM, Lim JJ, Yoo CG, Lee CT, Han SK, Shim YS, et al. The role of uteroglobin in the immunomodulation of nonsmall cell lung cancer cells. Tuberc Respir Dis. 2004. 57:336–344.7. Grosch S, Maier TJ, Schiffmann S, Geisslinger G. Cyclooxygenase-2 (COX-2)-independent anticarcinogenic effects of selective COX-2 inhibitors. J Natl Cancer Inst. 2006. 98:736–747.8. Cervello M, Montalto G. Cyclooxygenases in hepatocellular carcinoma. World J Gastroenterol. 2006. 12:5113–5121.9. Clay FJ, McEwen SJ, Bertoncello I, Wilks AF, Dunn AR. Identification and cloning of a protein kinase-encoding mouse gene, Plk, related to the polo gene of Drosophila. Proc Natl Acad Sci U S A. 1993. 90:4882–4886.10. Sunkel CE, Glover DM. polo, a mitotic mutant of Drosophila displaying abnormal spindle poles. J Cell Sci. 1988. 89:25–38.11. Nigg EA. Polo-like kinases: positive regulators of cell division from start to finish. Curr Opin Cell Biol. 1998. 10:776–783.12. Donaldson MM, Tavares AA, Hagan IM, Nigg EA, Glover DM. The mitotic roles of Polo-like kinase. J Cell Sci. 2001. 114:2357–2358.13. Wolf G, Elez R, Doermer A, Holtrich U, Ackermann H, Stutte HJ, et al. Prognostic significance of polo-like kinase (PLK) expression in non-small cell lung cancer. Oncogene. 1997. 14:543–549.14. Tokumitsu Y, Mori M, Tanaka S, Akazawa K, Nakano S, Niho Y. Prognostic significance of polo-like kinase expression in esophageal carcinoma. Int J Oncol. 1999. 15:687–692.15. Kneisel L, Strebhardt K, Bernd A, Wolter M, Binder A, Kaufmann R. Expression of polo-like kinase (PLK1) in thin melanomas: a novel marker of metastatic disease. J Cutan Pathol. 2002. 29:354–358.16. Wolf G, Hildenbrand R, Schwar C, Grobholz R, Kaufmann M, Stutte HJ, et al. Polo-like kinase: a novel marker of proliferation: correlation with estrogen-receptor expression in human breast cancer. Pathol Res Pract. 2000. 196:753–759.17. Dietzmann K, Kirches E, von B, Jachau K, Mawrin C. Increased human polo-like kinase-1 expression in gliomas. J Neurooncol. 2001. 53:1–11.18. Takai N, Miyazaki T, Fujisawa K, Nasu K, Hamanaka R, Miyakawa I. Expression of polo-like kinase in ovarian cancer is associated with histological grade and clinical stage. Cancer Lett. 2001. 164:41–49.19. Takai N, Miyazaki T, Fujisawa K, Nasu K, Hamanaka R, Miyakawa I. Polo-like kinase (PLK) expression in endometrial carcinoma. Cancer Lett. 2001. 169:41–49.20. Kawata E, Ashihara E, Kimura S, Takenaka K, Sato K, Tanaka R, et al. Administration of PLK-1 small interfering RNA with atelocollagen prevents the growth of liver metastases of lung cancer. Mol Cancer Ther. 2008. 7:2904–2912.21. Denkert C, Thoma A, Niesporek S, Weichert W, Koch I, Noske A, et al. Overexpression of cyclooxygenase-2 in human prostate carcinoma and prostatic intraepithelial neoplasia-association with increased expression of Polo-like kinase-1. Prostate. 2007. 67:361–369.22. Fosslien E. Molecular pathology of cyclooxygenase-2 in neoplasia. Ann Clin Lab Sci. 2000. 30:3–21.23. Jang JW. Anti-tumor mechanisms and regulation of survivin by selective cyclooxygenase-2 inhibitor. Korean J Hepatol. 2008. 14:305–308.24. Peri A, Cordella-Miele E, Miele L, Mukherjee AB. Tissue-specific expression of the gene coding for human Clara cell 10-kD protein, a phospholipase A2-inhibitory protein. J Clin Invest. 1993. 92:2099–2109.25. Yoon JH, Kim KS, Kim HU, Linton JA, Lee JG. Effects of TNF-alpha and IL-1 beta on mucin, lysozyme, IL-6 and IL-8 in passage-2 normal human nasal epithelial cells. Acta Otolaryngol. 1999. 119:905–910.26. Shelhamer JH, Levine SJ, Wu T, Jacoby DB, Kaliner MA, Rennard SI. NIH conference. Airway inflammation. Ann Intern Med. 1995. 123:288–304.27. Lin CH, Sheu SY, Lee HM, Ho YS, Lee WS, Ko WC, et al. Involvement of protein kinase C-gamma in IL-1beta-induced cyclooxygenase-2 expression in human pulmonary epithelial cells. Mol Pharmacol. 2000. 57:36–43.28. Morton RS, Dongari-Bagtzoglou AI. Cyclooxygenase-2 is upregulated in inflamed gingival tissues. J Periodontol. 2001. 72:461–469.29. Laporte JD, Moore PE, Panettieri RA, Moeller W, Heyder J, Shore SA. Prostanoids mediate IL-1beta-induced beta-adrenergic hyporesponsiveness in human airway smooth muscle cells. Am J Physiol. 1998. 275:L491–L501.30. Pang L, Knox AJ. Effect of interleukin-1 beta, tumour necrosis factor-alpha and interferon-gamma on the induction of cyclo-oxygenase-2 in cultured human airway smooth muscle cells. Br J Pharmacol. 1997. 121:579–587.31. Strakova Z, Srisuparp S, Fazleabas AT. Interleukin-1beta induces the expression of insulin-like growth factor binding protein-1 during decidualization in the primate. Endocrinology. 2000. 141:4664–4670.32. Kim YD, Song SY, Kwon EJ, Baek SH, Cho GS, Kim HS, et al. IL-1beta mediated COX-2 expression in human airway epithelial cells. Korean J Otolaryngol - Head Neck Surg. 2002. 45:132–136.33. Pyrko P, Soriano N, Kardosh A, Liu YT, Uddin J, Petasis NA, et al. Downregulation of survivin expression and concomitant induction of apoptosis by celecoxib and its non-cyclooxygenase-2-inhibitory analog, dimethyl-celecoxib (DMC), in tumor cells in vitro and in vivo. Mol Cancer. 2006. 5:19.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Overview of ALK and ROS1 Rearranged Lung Cancer
- Expressions of CD44s Is Associated with the Expression of Cyclooxygenase-2 in Non-Small Cell Lung Cancers
- Involvement of Mitogen-Activated Protein Kinase in Aspirin-induced Expression of Cyclooxygenase-2 in Amnionic Cell Line WISH Cells
- Druggable Targets of Squamous Cell Lung Cancer
- Adjuvant Chemotherapy for Completely Resected Non-Small Cell Lung Cancer