Nutr Res Pract.
2017 Aug;11(4):265-274. 10.4162/nrp.2017.11.4.265.
Anti-inflammatory effects of Nelumbo leaf extracts and identification of their metabolites
- Affiliations
-
- 1Department of Home Economics Education, Chung-Ang University, Seoul 06974, Korea. jjhkim@cau.ac.kr
- 2Department of Food Science & Technology, Chung-Ang University, 4726, Seodong-daero, Daedeok-myeon, Anseong, Gyeonggi 17546, Korea. jhauh@cau.ac.kr
- 3Department of Physical Education, Chung-Ang University, 84, Heukseok-ro, Dongjak-gu, Seoul 06974, Korea.
- KMID: 2385464
- DOI: http://doi.org/10.4162/nrp.2017.11.4.265
Abstract
- BACKGROUND/OBJECTIVES
Nelumbo leaves have been used in traditional medicine to treat bleeding, gastritis, hemorrhoids, and halitosis. However, their mechanisms have not been elucidated.
MATERIALS/METHODS
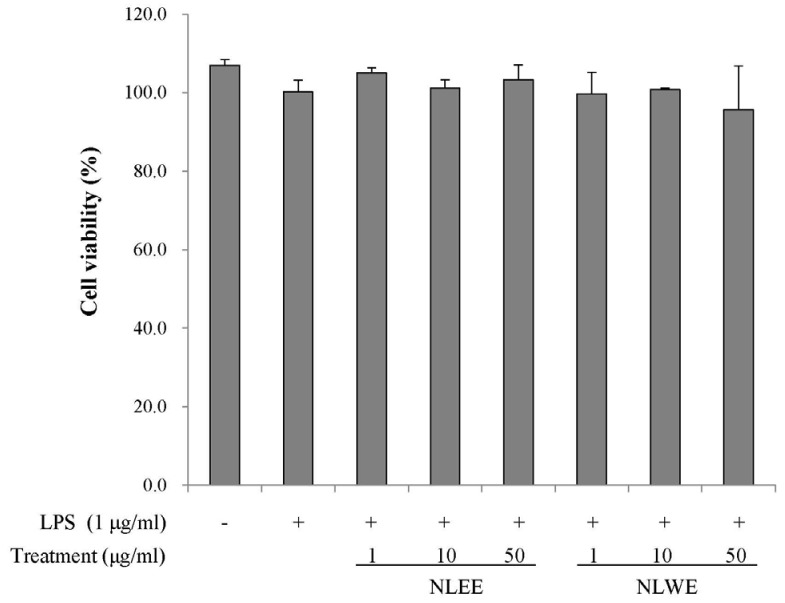
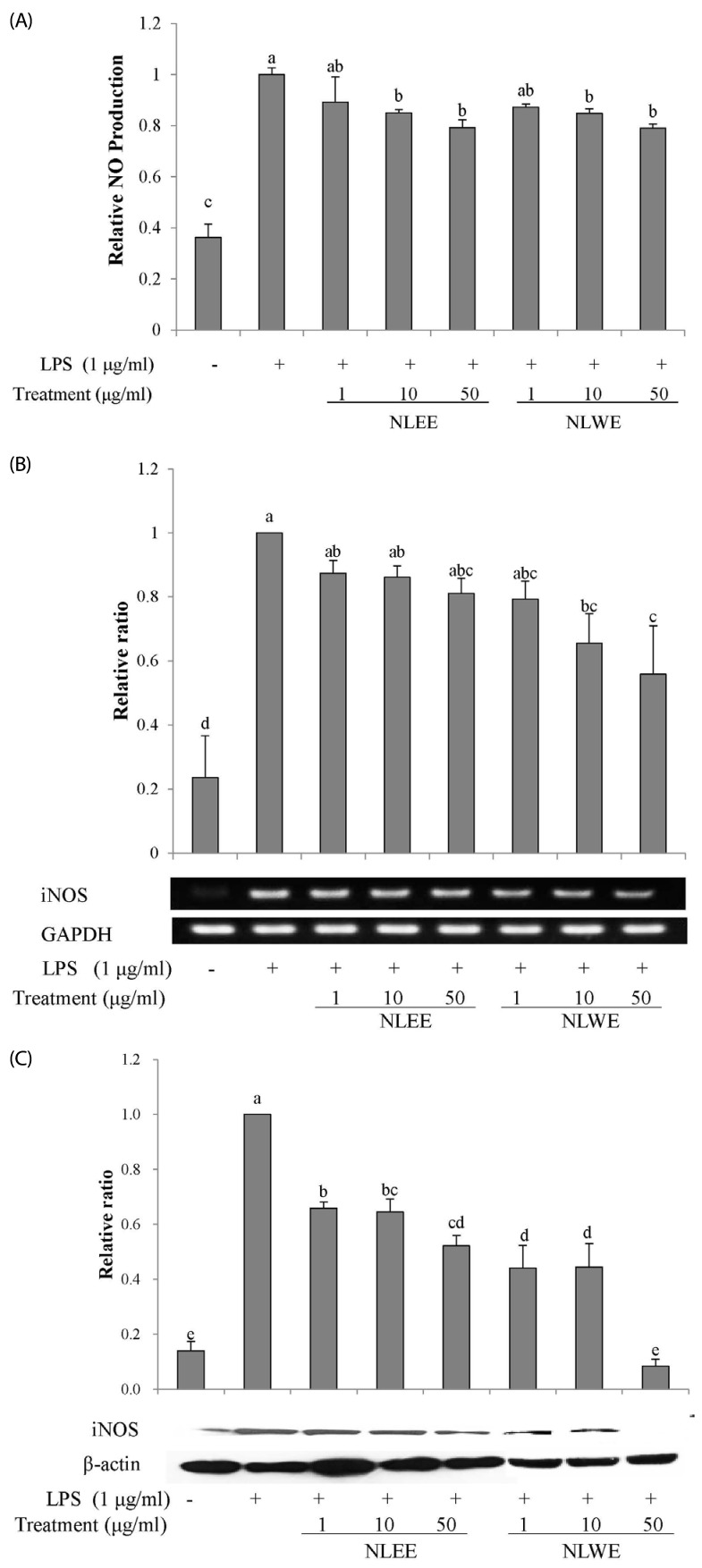
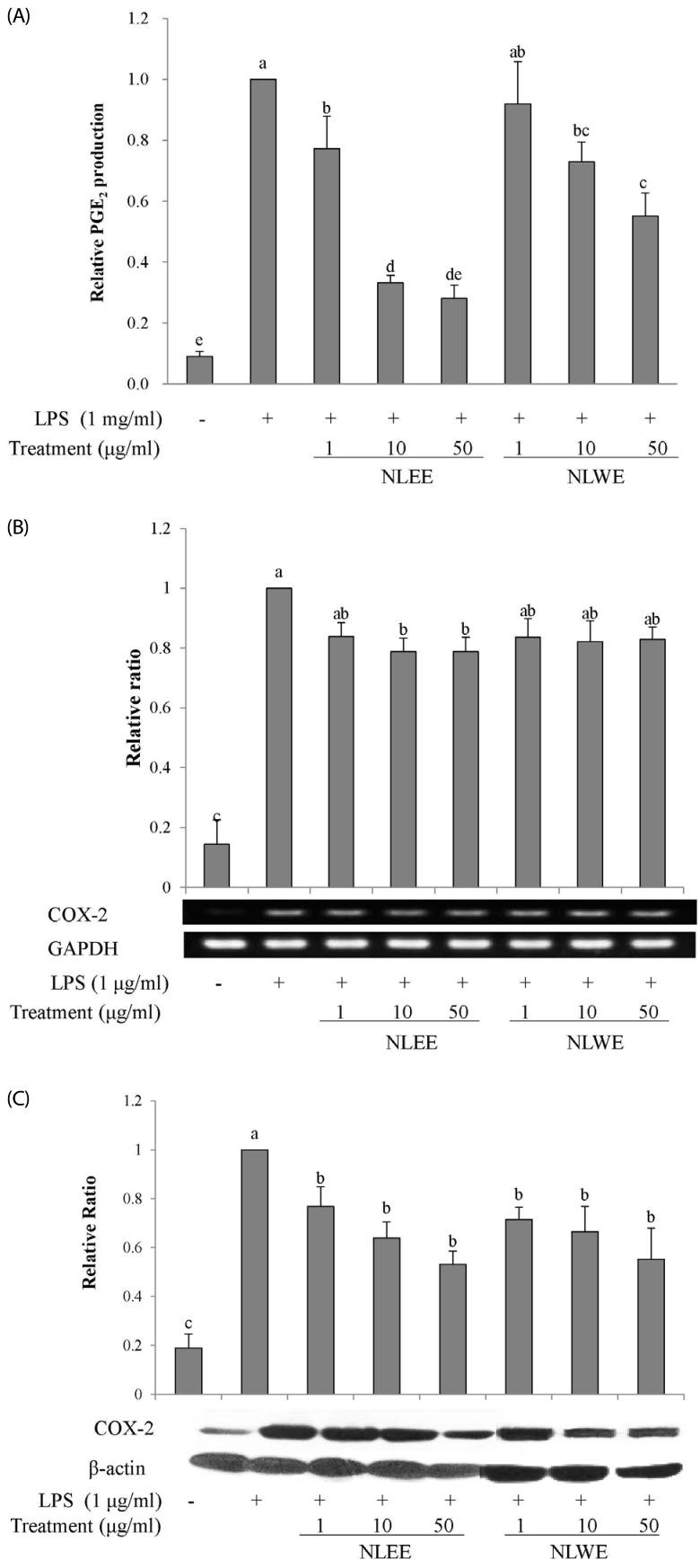
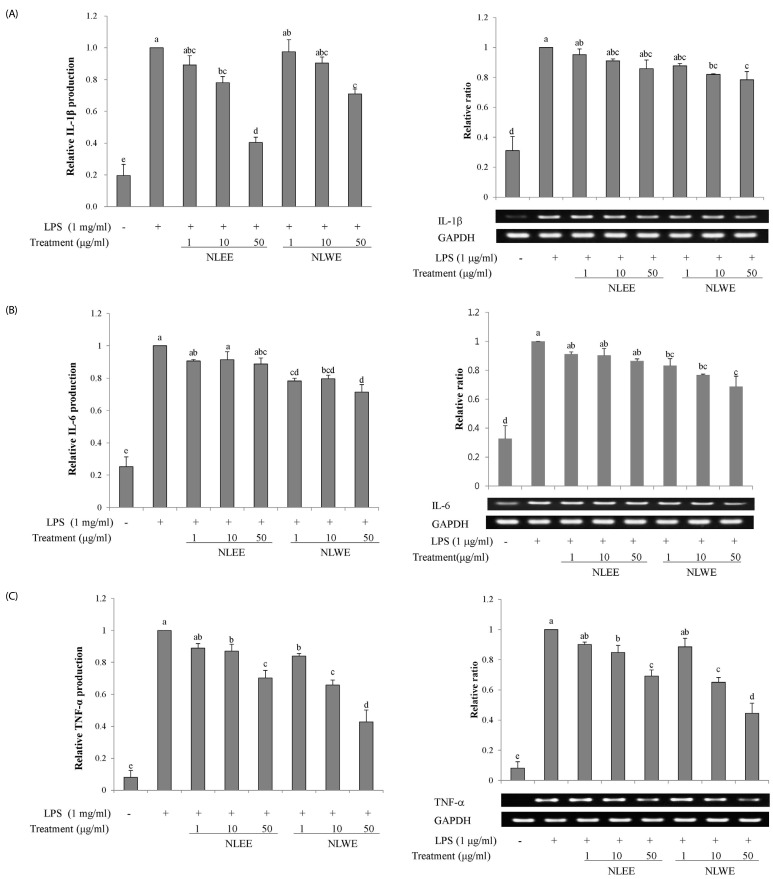
The present study prepared two Nelumbo leaf extracts (NLEs) using water or 50% ethanol. Inflammatory response was induced with LPS treatment, and expression of pro-inflammatory mediators (inducible nitric oxide synthase, cyclooxygenase-2, tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and IL-6 and nitric oxide (NO) and prostaglandin E₂ (PGE₂) productions were assessed. To determine the anti-inflammatory mechanism of NLEs, we measured nuclear factor-κB (NF-κB) activity. Major metabolites of NLEs were also analyzed and quantified.
RESULTS
NLEs effectively reduced the expression and productions of pro-inflammatory mediators such as IL-1β, IL-6, TNF-α, PGE₂, and NO. NLEs also reduced NF-κB activity by inhibiting inhibitor of NF-κB phosphorylation. Both extracts contained catechin and quercetin, bioactive compounds of NLEs.
CONCLUSIONS
In this study, we showed that NLEs could be used to inhibit NF-κB-mediated inflammatory responses. In addition, our data support the idea that NLEs can ameliorate disease conditions involving chronic inflammation.
Keyword
MeSH Terms
-
Catechin
Cyclooxygenase 2
Dinoprostone
Ethanol
Gastritis
Halitosis
Hemorrhage
Hemorrhoids
Inflammation
Interleukin-6
Interleukins
Macrophages
Medicine, Traditional
Metabolomics
Necrosis
Nelumbo*
Nitric Oxide
Nitric Oxide Synthase
Phosphorylation
Quercetin
Water
Catechin
Cyclooxygenase 2
Dinoprostone
Ethanol
Interleukin-6
Interleukins
Nitric Oxide
Nitric Oxide Synthase
Quercetin
Water
Figure
Reference
-
1. Hunter M, Wang Y, Eubank T, Baran C, Nana-Sinkam P, Marsh C. Survival of monocytes and macrophages and their role in health and disease. Front Biosci (Landmark Ed). 2009; 14:4079–4102. PMID: 19273336.
Article2. Laroux FS. Mechanisms of inflammation: the good, the bad and the ugly. Front Biosci. 2004; 9:3156–3162. PMID: 15353345.
Article3. Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996; 87:13–20. PMID: 8858144.4. Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005; 4:281–286. PMID: 16101534.
Article5. Aggarwal BB, Gehlot P. Inflammation and cancer: how friendly is the relationship for cancer patients? Curr Opin Pharmacol. 2009; 9:351–369. PMID: 19665429.
Article6. McGeer PL, McGeer EG. Inflammation and the degenerative diseases of aging. Ann N Y Acad Sci. 2004; 1035:104–116. PMID: 15681803.
Article7. Liao CH, Lin JY. Purification, partial characterization and anti-inflammatory characteristics of lotus (Nelumbo nucifera Gaertn) plumule polysaccharides. Food Chem. 2012; 135:1818–1827. PMID: 22953928.
Article8. Ahn JH, Kim ES, Lee C, Kim S, Cho SH, Hwang BY, Lee MK. Chemical constituents from Nelumbo nucifera leaves and their anti-obesity effects. Bioorg Med Chem Lett. 2013; 23:3604–3608. PMID: 23642481.
Article9. Mukherjee PK, Mukherjee D, Maji AK, Rai S, Heinrich M. The sacred lotus (Nelumbo nucifera) - phytochemical and therapeutic profile. J Pharm Pharmacol. 2009; 61:407–422. PMID: 19298686.10. Sridhar KR, Bhat R. Lotus: a potential nutraceutical source. J Agric Technol. 2007; 3:143–155.11. Bensky D, Clavey S, Stoger E. Chinese Herbal Medicine: Materia Medica. 3rd ed. Seattle (WA): Eastland Press;2015.12. Li M, Xu Z. Quercetin in a lotus leaves extract may be responsible for antibacterial activity. Arch Pharm Res. 2008; 31:640–644. PMID: 18481022.
Article13. Yang D, Wang Q, Ke L, Jiang J, Ying T. Antioxidant activities of various extracts of lotus (Nelumbo nuficera Gaertn) rhizome. Asia Pac J Clin Nutr. 2007; 16(Suppl 1):158–163.14. Lin HY, Kuo YH, Lin YL, Chiang W. Antioxidative effect and active components from leaves of Lotus (Nelumbo nucifera). J Agric Food Chem. 2009; 57:6623–6629. PMID: 19572539.
Article15. Mukherjee PK, Saha K, Das J, Pal M, Saha BP. Studies on the anti-inflammatory activity of rhizomes of Nelumbo nucifera. Planta Med. 1997; 63:367–369. PMID: 9270384.16. Liu CP, Tsai WJ, Lin YL, Liao JF, Chen CF, Kuo YC. The extracts from Nelumbo Nucifera suppress cell cycle progression, cytokine genes expression, and cell proliferation in human peripheral blood mononuclear cells. Life Sci. 2004; 75:699–716. PMID: 15172179.
Article17. Ono Y, Hattori E, Fukaya Y, Imai S, Ohizumi Y. Anti-obesity effect of Nelumbo nucifera leaves extract in mice and rats. J Ethnopharmacol. 2006; 106:238–244. PMID: 16495025.
Article18. Tsuruta Y, Nagao K, Kai S, Tsuge K, Yoshimura T, Koganemaru K, Yanagita T. Polyphenolic extract of lotus root (edible rhizome of Nelumbo nucifera) alleviates hepatic steatosis in obese diabetic db/db mice. Lipids Health Dis. 2011; 10:202. PMID: 22067945.
Article19. Chen S, Fang L, Xi H, Guan L, Fang J, Liu Y, Wu B, Li S. Simultaneous qualitative assessment and quantitative analysis of flavonoids in various tissues of lotus (Nelumbo nucifera) using high performance liquid chromatography coupled with triple quad mass spectrometry. Anal Chim Acta. 2012; 724:127–135. PMID: 22483220.
Article20. Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: an overview. ScientificWorldJournal. 2013; 2013:162750. PMID: 24470791.
Article21. Bino RJ, Hall RD, Fiehn O, Kopka J, Saito K, Draper J, Nikolau BJ, Mendes P, Roessner-Tunali U, Beale MH, Trethewey RN, Lange BM, Wurtele ES, Sumner LW. Potential of metabolomics as a functional genomics tool. Trends Plant Sci. 2004; 9:418–425. PMID: 15337491.
Article22. Huang B, Ban X, He J, Tong J, Tian J, Wang Y. Comparative analysis of essential oil components and antioxidant activity of extracts of Nelumbo nucifera from various areas of China. J Agric Food Chem. 2010; 58:441–448. PMID: 19919095.
Article23. Zhu MZ, Wu W, Jiao LL, Yang PF, Guo MQ. Analysis of flavonoids in lotus (Nelumbo nucifera) leaves and their antioxidant activity using macroporous resin chromatography coupled with LC-MS/MS and antioxidant biochemical assays. Molecules. 2015; 20:10553–10565. PMID: 26060918.
Article24. Naowaratwattana W, De-Eknamkul W, De Mejia EG. Phenolic-containing organic extracts of mulberry (Morus alba L.) leaves inhibit HepG2 hepatoma cells through G2/M phase arrest, induction of apoptosis, and inhibition of topoisomerase IIalpha activity. J Med Food. 2010; 13:1045–1056. PMID: 20828312.25. Cuevas-Valenzuela J, González-Rojas A, Wisniak J, Apelblat A, Pérez-Correa JR. Solubility of (+)-catechin in water and water-ethanol mixtures within the temperature range 277.6-331.2 K: fundamental data to design polyphenol extraction processes. Fluid Phase Equilib. 2014; 382:279–285.26. Auclair S, Milenkovic D, Besson C, Chauvet S, Gueux E, Morand C, Mazur A, Scalbert A. Catechin reduces atherosclerotic lesion development in apo E-deficient mice: a transcriptomic study. Atherosclerosis. 2009; 204:e21–e27. PMID: 19152914.
Article27. Mangiapane H, Thomson J, Salter A, Brown S, Bell GD, White DA. The inhibition of the oxidation of low density lipoprotein by (+)-catechin, a naturally occurring flavonoid. Biochem Pharmacol. 1992; 43:445–450. PMID: 1540202.
Article28. Weyant MJ, Carothers AM, Dannenberg AJ, Bertagnolli MM. (+)-Catechin inhibits intestinal tumor formation and suppresses focal adhesion kinase activation in the min/+ mouse. Cancer Res. 2001; 61:118–125. PMID: 11196148.29. Handa O, Naito Y, Takagi T, Ishikawa T, Ueda M, Matsumoto N, Kokura S, Ichikawa H, Yoshida N, Shimoi K, Yoshikawa T. Inhibitory effects of catechins on neutrophil-dependent gastric inflammation. Redox Rep. 2002; 7:324–328. PMID: 12688521.
Article30. Iijima T, Mohri Y, Hattori Y, Kashima A, Kamo T, Hirota M, Kiyota H, Makabe H. Synthesis of (-)-epicatechin 3-(3-O-methylgallate) and (+)-catechin 3-(3-O-methylgallate), and their anti-inflammatory activity. Chem Biodivers. 2009; 6:520–526. PMID: 19353533.
Article31. Monga J, Aggarwal V, Suthar SK, Monika , Nongalleima K, Sharma M. Topical (+)-catechin emulsified gel prevents DMBA/TPA-induced squamous cell carcinoma of the skin by modulating antioxidants and inflammatory biomarkers in BALB/c mice. Food Funct. 2014; 5:3197–3207. PMID: 25310126.
Article32. Ademosun AO, Oboh G, Bello F, Ayeni PO. Antioxidative properties and effect of quercetin and its glycosylated form (Rutin) on acetylcholinesterase and butyrylcholinesterase activities. J Evid Based Complementary Altern Med. 2016; 21:NP11–NP17. PMID: 26438716.
Article33. Endale M, Park SC, Kim S, Kim SH, Yang Y, Cho JY, Rhee MH. Quercetin disrupts tyrosine-phosphorylated phosphatidylinositol 3-kinase and myeloid differentiation factor-88 association, and inhibits MAPK/AP-1 and IKK/NF-kappaB-induced inflammatory mediators production in RAW 264.7 cells. Immunobiology. 2013; 218:1452–1467. PMID: 23735482.34. Maurya AK, Vinayak M. Anticarcinogenic action of quercetin by downregulation of phosphatidylinositol 3-kinase (PI3K) and protein kinase C (PKC) via induction of p53 in hepatocellular carcinoma (HepG2) cell line. Mol Biol Rep. 2015; 42:1419–1429. PMID: 26311153.
Article35. Ahn JY, Choi SE, Jeong MS, Park KH, Moon NJ, Joo SS, Lee CS, Choi YW, Li K, Lee MK, Lee MW, Seo SJ. Effect of taxifolin glycoside on atopic dermatitis-like skin lesions in NC/Nga mice. Phytother Res. 2010; 24:1071–1077. PMID: 20041431.
Article36. Ince I, Yesil-Celiktas O, Karabay-Yavasoglu NU, Elgin G. Effects of Pinus brutia bark extract and Pycnogenol in a rat model of carrageenan induced inflammation. Phytomedicine. 2009; 16:1101–1104. PMID: 19577447.
Article37. Kwon JH, Kim SB, Park KH, Lee MW. Antioxidative and anti-inflammatory effects of phenolic compounds from the roots of Ulmus macrocarpa. Arch Pharm Res. 2011; 34:1459–1466. PMID: 21975807.
Article38. Wang YH, Wang WY, Chang CC, Liou KT, Sung YJ, Liao JF, Chen CF, Chang S, Hou YC, Chou YC, Shen YC. Taxifolin ameliorates cerebral ischemia-reperfusion injury in rats through its anti-oxidative effect and modulation of NF-kappa B activation. J Biomed Sci. 2006; 13:127–141. PMID: 16283433.
Article39. Sung J, Lee J. Anti-inflammatory activity of butein and luteolin through suppression of NFκB activation and induction of Heme Oxygenase-1. J Med Food. 2015; 18:557–564. PMID: 25692285.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-inflammatory effects of ethanolic extract of Annona muricata
- Anti-inflammatory effects of fruit and leaf extracts of Lycium barbarum in lipopolysaccharide-stimulated RAW264.7 cells and animal model
- Fermented Product Extract with Lentinus edodes Attenuate the Inflammatory Mediators Releases and Free Radical Production
- Nelumbo nucifera Leaf Extract Regulates Lipid Metabolism and Differentiation in 3T3-L1 Adipocytes and db/db Mice
- Anti-Inflammatory Herbal Extracts and Their Drug Discovery Perspective in Atopic Dermatitis