Korean J Gastroenterol.
2016 Oct;68(4):186-194. 10.4166/kjg.2016.68.4.186.
Role of Inhibitory Transforming Growth Factor-β Signal Smad7 in Helicobacter pylori-associated Gastric Damage
- Affiliations
-
- 1Department of Biochemistry, Gachon University School of Medicine, Incheon, Korea.
- 2CHA Cancer Prevention Research Center, CHA Bio Complex, CHA University, Seongnam, Korea. hahmkb@cha.ac.kr
- 3Digestive Disease Center, CHA Bundang Medical Center, Seongnam, Korea.
- KMID: 2383514
- DOI: http://doi.org/10.4166/kjg.2016.68.4.186
Abstract
- BACKGROUND/AIMS
Transforming growth factor-beta (TGF-β) is a cytokine implicated in the susceptibility, development, and progression of gastrointestinal cancer and certain other neoplasms. In the later stages of cancer, TGF-β not only acts as a bystander of host-immune response, but also contributes to cell growth, invasion, and metastasis. In the current study, we generated gastric mucosal cells that stably express Smad7, and explored the Helicobacter pylori-associated biological changes between mock-transfected and Smad7-transfected RGM1 cells.
METHODS
RGM1 cells stably transfected with Smad7 were infected with H. pylori, and molecular changes in apoptotic markers and inflammatory mediators were examined. Several candidate genes were explored in Smad7-overexpressing cells after H. pylori infection.
RESULTS
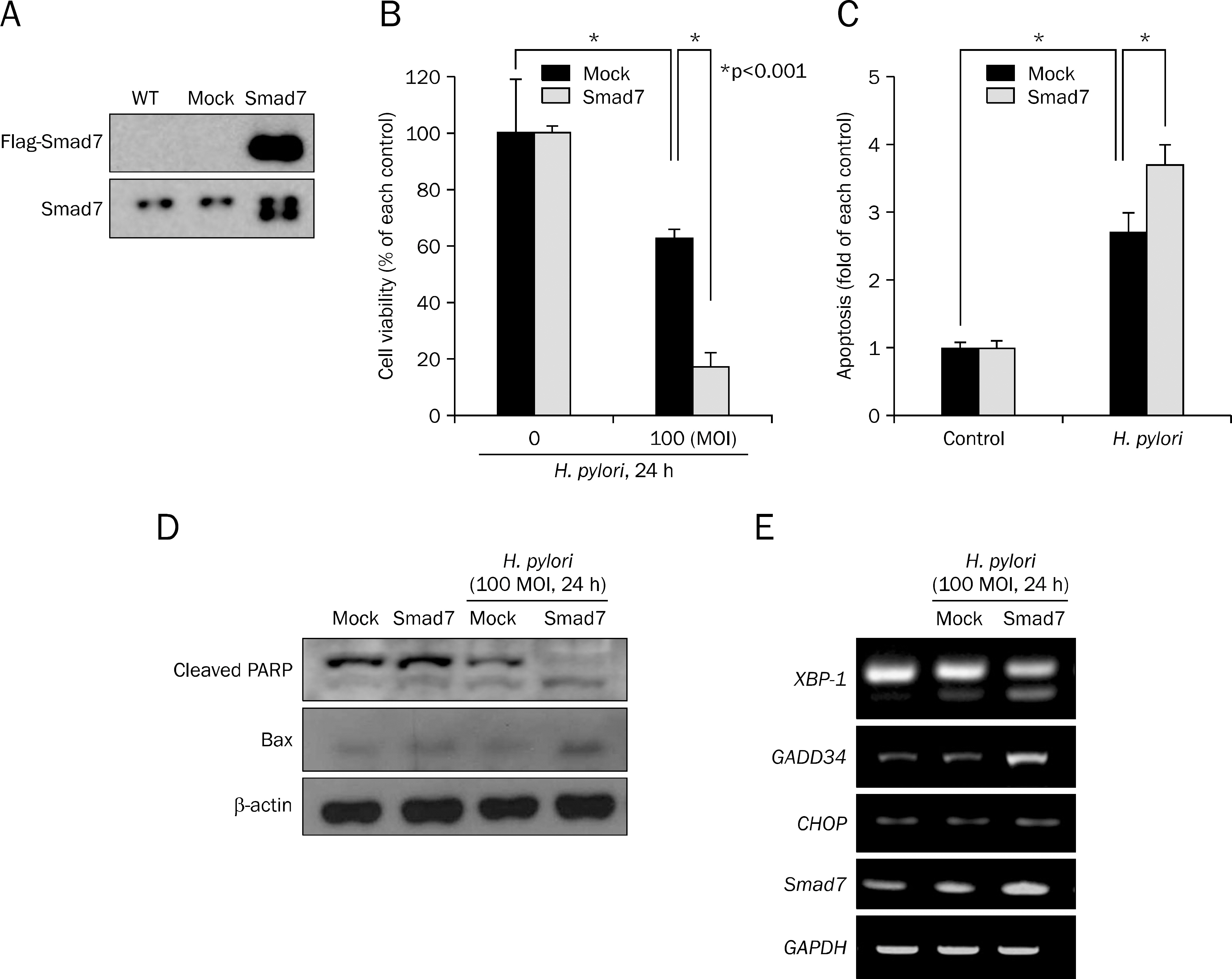
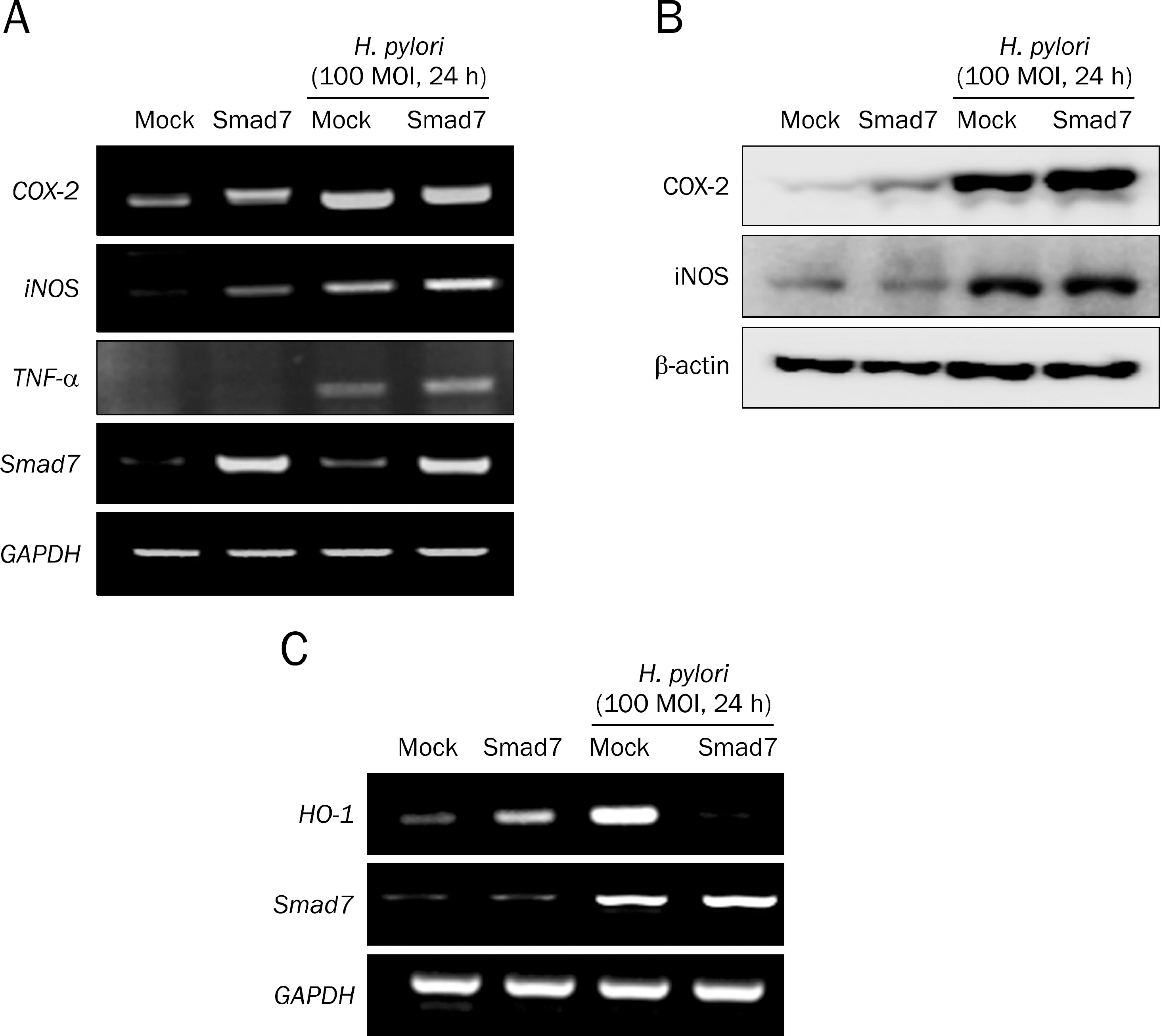
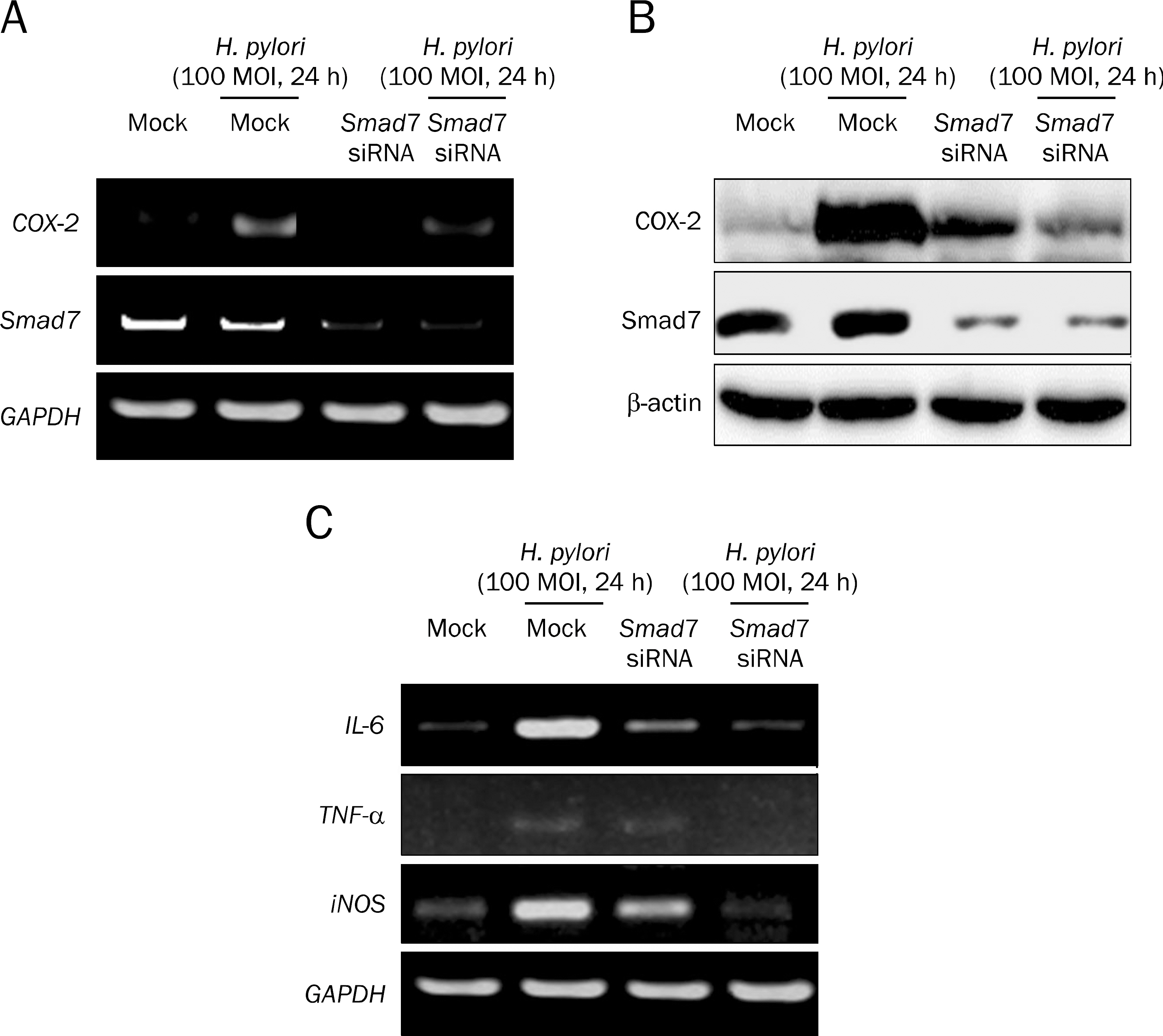
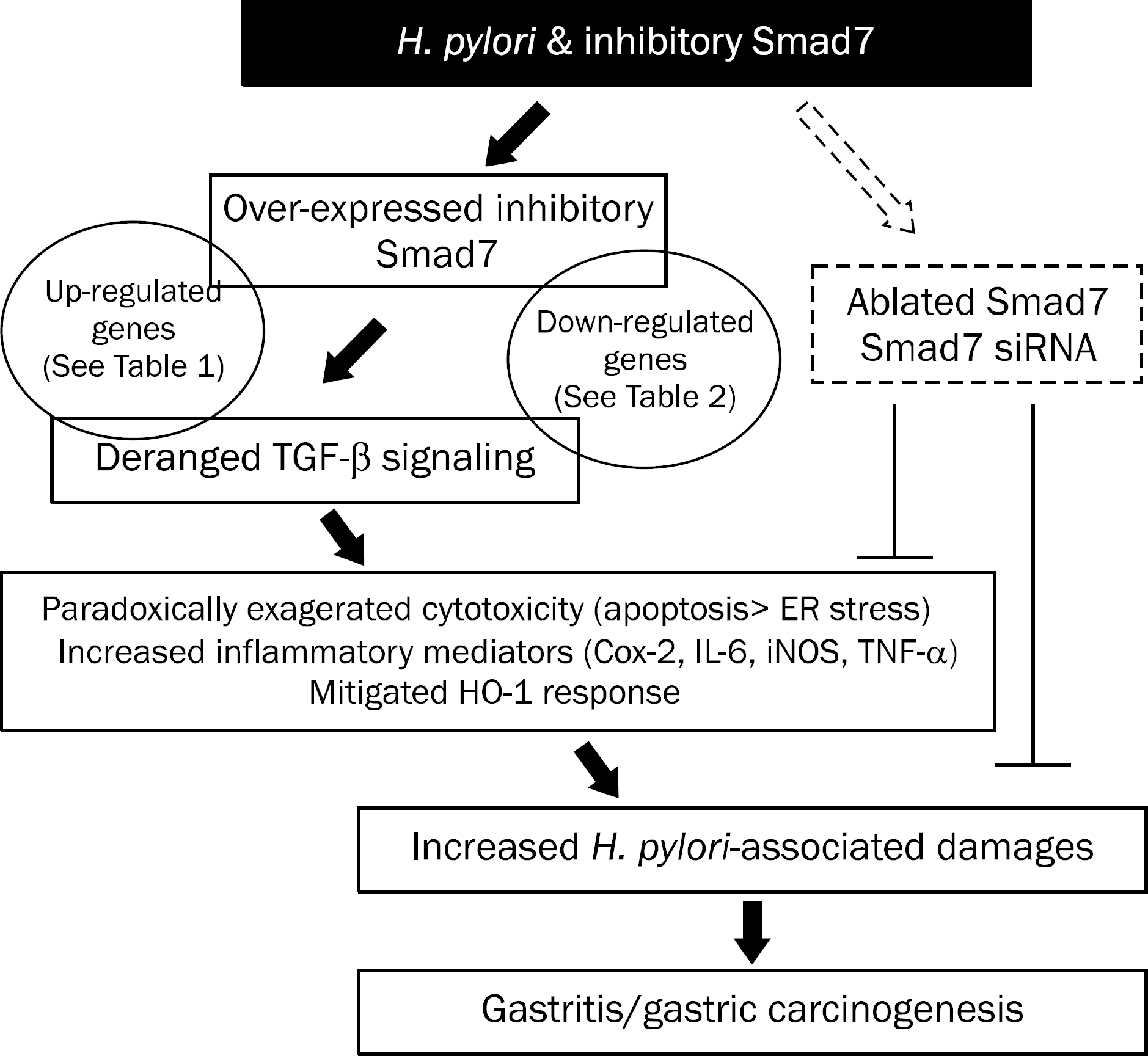
Overexpression of Smad7 in RGM1 cells significantly increased the H. pylori-induced cytotoxicity compared to mock-transfected cells. Exaggerated increases in inflammatory mediators, cyclooxygenase 2, inducible NO synthase, and augmented apoptosis were noted in Smad7-overexpressing cells, whereas mitigated heme oxygenase 1 was noted in Smad7- overexpressing cells. These phenomena were reversed in cells transfected with Smad7 siRNA.
CONCLUSIONS
These data suggest that inhibition of Smad7 is a possible target for mitigating H. pylori-associated inflammation.
MeSH Terms
-
Apoptosis
Cyclooxygenase 2
Gastritis
Gastrointestinal Neoplasms
Helicobacter pylori
Helicobacter*
Heme Oxygenase-1
Inflammation
Neoplasm Metastasis
Nitric Oxide Synthase
RNA, Small Interfering
Transforming Growth Factor beta
Cyclooxygenase 2
Heme Oxygenase-1
Nitric Oxide Synthase
RNA, Small Interfering
Transforming Growth Factor beta
Figure
Reference
-
References
1. Im YH, Kim HT, Kim IY, et al. Heterozygous mice for the transforming growth factor-beta type II receptor gene have increased susceptibility to hepatocellular carcinogenesis. Cancer Res. 2001; 61:6665–6668.2. Hahm KB, Cho K, Lee C, et al. Repression of the gene encoding the TGFbeta type II receptor is a major target of the EWS-FLI1 oncoprotein. Nat Genet. 1999; 23:222–227.3. Hahm KB, Lee KM, Kim YB, et al. Conditional loss of TGFbeta signalling leads to increased susceptibility to gastrointestinal carcinogenesis in mice. Aliment Pharmacol Ther. 2002; 16(Suppl 2):115–127.4. Hahm KB, Im YH, Parks TW, et al. Loss of transforming growth factor beta signalling in the intestine contributes to tissue injury in inflammatory bowel disease. Gut. 2001; 49:190–198.5. Hahm KB, Im YH, Lee C, et al. Loss of TGFbeta signaling contributes to autoimmune pancreatitis. J Clin Invest. 2000; 105:1057–1065.6. Camilo V, Barros R, Sousa S, et al. Helicobacter pylori and the BMP pathway regulate CDX2 and SOX2 expression in gastric cells. Carcinogenesis. 2012; 33:1985–1992.7. Shiotani A, Kamada T, Yamanaka Y, et al. Sonic hedgehog and CDX2 expression in the stomach. J Gastroenterol Hepatol. 2008; 23(Suppl 2):S161–S166.
Article8. Lee KM, Lee JS, Jung HS, Park DK, Park HS, Hahm KB. Late reactivation of sonic hedgehog by Helicobacter pylori results in population of gastric epithelial cells that are resistant to apoptosis: implication for gastric carcinogenesis. Cancer Lett. 2010; 287:44–53.9. Barros R, Pereira B, Duluc I, et al. Key elements of the BMP/SMAD pathway co-localize with CDX2 in intestinal metaplasia and regulate CDX2 expression in human gastric cell lines. J Pathol. 2008; 215:411–420.
Article10. Nguyen TT, Kim SJ, Park JM, Hahm KB, Lee HJ. Repressed TGF-signaling through CagA-Smad3 interaction as pathogenic mechanisms of Helicobacter pylori-associated gastritis. J Clin Biochem Nutr. 2015; 57:113–120.11. Pickup M, Novitskiy S, Moses HL. The roles of TGFin the tumour microenvironment. Nat Rev Cancer. 2013; 13:788–799.12. Wakefield LM, Hill CS. Beyond TGF: roles of other TGFsuper-family members in cancer. Nat Rev Cancer. 2013; 13:328–341.13. Akhurst RJ, Hata A. Targeting the TGFsignalling pathway in disease. Nat Rev Drug Discov. 2012; 11:790–811.14. Ikushima H, Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nat Rev Cancer. 2010; 10:415–424.15. Park S, Kim WS, Choi UJ, et al. Amelioration of oxidative stress with ensuing inflammation contributes to chemoprevention of H. pylori-associated gastric carcinogenesis. Antioxid Redox Signal. 2004; 6:549–560.16. Han SU, Kim YB, Joo HJ, et al. Helicobacter pylori infection promotes gastric carcinogenesis in a mice model. J Gastroenterol Hepatol. 2002; 17:253–261.17. Schmierer B, Hill CS. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007; 8:970–982.18. Yingling JM, Blanchard KL, Sawyer JS. Development of TGFbeta signalling inhibitors for cancer therapy. Nat Rev Drug Discov. 2004; 3:1011–1022.19. Siegel PM, Massagué J. Cytostatic and apoptotic actions of TGFbeta in homeostasis and cancer. Nat Rev Cancer. 2003; 3:807–821.20. Hong S, Lim S, Li AG, et al. Smad7 binds to the adaptors TAB2 and TAB3 to block recruitment of the kinase TAK1 to the adaptor TRAF2. Nat Immunol. 2007; 8:504–513.
Article21. Yan X, Chen YG. Smad7: not only a regulator, but also a crosstalk mediator of TGFsignalling. Biochem J. 2011; 434:1–10.22. Akazawa Y, Isomoto H, Matsushima K, et al. Endoplasmic reticulum stress contributes to Helicobacter pylori VacA-induced apoptosis. PLoS One. 2013; 8:e82322.23. Monteleone G, Neurath MF, Ardizzone S, et al. Mongersen, an oral SMAD7 antisense oligonucleotide, and Crohn's disease. N Engl J Med. 2015; 372:1104–1113.
Article24. Monteleone G, Di Sabatino A, Ardizzone S, et al. Impact of patient characteristics on the clinical efficacy of mongersen (GED-0301), an oral Smad7 antisense oligonucleotide, in active Crohn's disease. Aliment Pharmacol Ther. 2016; 43:717–724.
Article25. Ardizzone S, Bevivino G, Monteleone G. Mongersen, an oral Smad7 antisense oligonucleotide, in patients with active Crohn's disease. Therap Adv Gastroenterol. 2016; 9:527–532.
Article26. Monteleone G, Del Vecchio Blanco G, Palmieri G, et al. Induction and regulation of Smad7 in the gastric mucosa of patients with Helicobacter pylori infection. Gastroenterology. 2004; 126:674–682.27. Monteleone G, Kumberova A, Croft NM, McKenzie C, Steer HW, MacDonald TT. Blocking Smad7 restores TGFbeta1 signaling in chronic inflammatory bowel disease. J Clin Invest. 2001; 108:601–609.28. Monteleone G, Mann J, Monteleone I, et al. A failure of transforming growth factor-beta1 negative regulation maintains sustained NF-kappaB activation in gut inflammation. J Biol Chem. 2004; 279:3925–3932.29. Monteleone G, Pallone F, MacDonald TT. Smad7 in TGF-be-ta-mediated negative regulation of gut inflammation. Trends Immunol. 2004; 25:513–517.30. Monteleone G, Boirivant M, Pallone F, MacDonald TT. TGFbeta1 and Smad7 in the regulation of IBD. Mucosal Immunol. 2008; 1(Suppl 1):S50–S53.31. Monteleone G, Caruso R, Pallone F. Role of Smad7 in inflammatory bowel diseases. World J Gastroenterol. 2012; 18:5664–5668.
Article32. Wang W, Huang XR, Li AG, et al. Signaling mechanism of TGF-beta1 in prevention of renal inflammation: role of Smad7. J Am Soc Nephrol. 2005; 16:1371–1383.33. Boirivant M, Pallone F, Di Giacinto C, et al. Inhibition of Smad7 25. Ardizzone S, Bevivino G, Monteleone G. Mongersen, an oral Smad7 antisense oligonucleotide, in patients with active Crohn's disease. Therap Adv Gastroenterol. 2016; 9:527–532.34. Han G, Bian L, Li F, et al. Preventive and therapeutic effects of Smad7 on radiation-induced oral mucositis. Nat Med. 2013; 19:421–428.
Article35. Yuasa Y. Control of gut differentiation and intestinal-type gastric carcinogenesis. Nat Rev Cancer. 2003; 3:592–600.
Article36. Beswick EJ, Pinchuk IV, Earley RB, Schmitt DA, Reyes VE. Role of gastric epithelial cell-derived transforming growth factor beta in reduced CD4+ T cell proliferation and development of regulatory T cells during Helicobacter pylori infection. Infect Immun. 2011; 79:2737–2745.37. Yang YJ, Chuang CC, Yang HB, Lu CC, Sheu BS. Lactobacillus acid-ophilus ameliorates H. pylori-induced gastric inflammation by in-activating the Smad7 and NFκ B pathways. BMC Microbiol. 2012; 12:38.
Article38. Datta De D, Bhattacharjya S, Maitra M, et al. IL1B induced Smad 7 negatively regulates gastrin expression. PLoS One. 2011; 6:e14775.
Article39. Jo Y, Han SU, Kim YJ, et al. Suppressed gastric mucosal TGF-beta1 increases susceptibility to H. pylori-Induced gastric inflammation and ulceration: a stupid host defense response. Gut Liver. 2010; 4:43–53.40. Tsugawa H, Suzuki H, Saya H, et al. Reactive oxygen species-induced autophagic degradation of Helicobacter pylori CagA is specifically suppressed in cancer stem-like cells. Cell Host Microbe. 2012; 12:764–777.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differential Role of Transforming Growth Factor-beta in an Osteoarthritic or a Healthy Joint
- The Effect of Helicobacter pylori on Epidermal Growth Factor Receptor-Induced Signal Transduction and the Preventive Effect of Celecoxib in Gastric Cancer Cells
- Helicobacter pylori-associated Chronic Atrophic Gastritis and Progression of Gastric Carcinogenesis
- Prevention of Gastric Cancer: Helicobacter pylori Treatment
- Suppression of Helicobacter pylori-induced Angiogenesis by a Gastric Proton Pump Inhibitor