Immune Netw.
2017 Jun;17(3):144-151. 10.4110/in.2017.17.3.144.
TLR/MyD88-mediated Innate Immunity in Intestinal Graft-versus-Host Disease
- Affiliations
-
- 1Department of Biomedical Sciences, Seoul National University College of Medicine, Seoul 03080, Korea. eycii@snu.ac.kr
- 2BioMembrane Plasticity Research Center (MPRC), Seoul National University College of Medicine, Seoul 03080, Korea.
- KMID: 2383352
- DOI: http://doi.org/10.4110/in.2017.17.3.144
Abstract
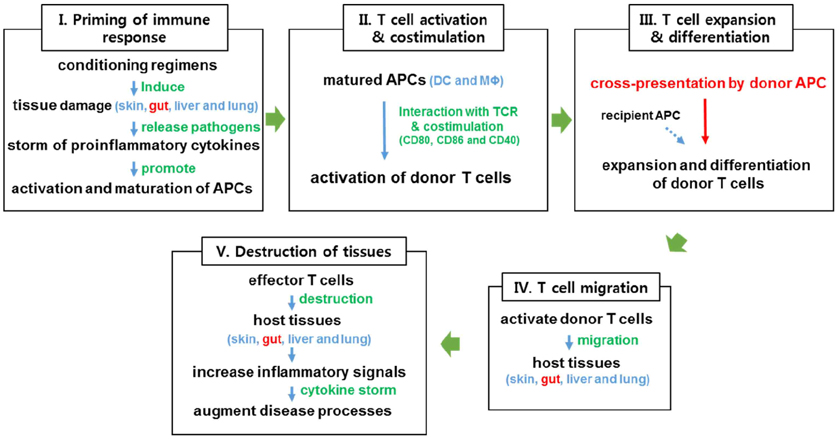
- Graft-versus-host disease (GHVD) is a severe complication after allogeneic hematopoietic stem cell transplantation. The degree of inflammation in the gastrointestinal tract, a major GVHD target organ, correlates with the disease severity. Intestinal inflammation is initiated by epithelial damage caused by pre-conditioning irradiation. In combination with damages caused by donor-derived T cells, such damage disrupts the epithelial barrier and exposes innate immune cells to pathogenic and commensal intestinal bacteria, which release ligands for Toll-like receptors (TLRs). Dysbiosis of intestinal microbiota and signaling through the TLR/myeloid differentiation primary response gene 88 (MyD88) pathways contribute to the development of intestinal GVHD. Understanding the changes in the microbial flora and the roles of TLR signaling in intestinal GVHD will facilitate the development of preventative and therapeutic strategies.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Skewed Dendritic Cell Differentiation of MyD88-Deficient Donor Bone Marrow Cells, Instead of Massive Expansion as Myeloid-Derived Suppressor Cells, Aggravates GVHD
Young-Kwan Lee, Ji-Min Ju, Woo-Jeong Shon, Sehwa Oh, Chang-Ki Min, Myung-Soo Kang, Dong-Mi Shin, Eun Young Choi
Immune Netw. 2018;18(6):. doi: 10.4110/in.2018.18.e44.
Reference
-
1. Mohty M, de LH, Ladaique P, Faucher C, Vey N, Coso D, Stoppa AM, Gastaut JA, Blaise D. The role of reduced intensity conditioning allogeneic stem cell transplantation in patients with acute myeloid leukemia: a donor vs no donor comparison. Leukemia. 2005; 19:916–920.
Article2. Poon LM, Bassett R Jr, Rondon G, Hamdi A, Qazilbash M, Hosing C, Jones RB, Shpall EJ, Popat UR, Nieto Y, Worth LL, Cooper L, De LM, Champlin RE, Kebriaei P. Outcomes of second allogeneic hematopoietic stem cell transplantation for patients with acute lymphoblastic leukemia. Bone Marrow Transplant. 2013; 48:666–670.
Article3. Demirer T, Barkholt L, Blaise D, Pedrazzoli P, Aglietta M, Carella AM, Bay JO, Arpaci F, Rosti G, Gurman G, Niederwieser D, Bregni M. Transplantation of allogeneic hematopoietic stem cells: an emerging treatment modality for solid tumors. Nat Clin Pract Oncol. 2008; 5:256–267.
Article4. Dvorak CC, Cowan MJ. Hematopoietic stem cell transplantation for primary immunodeficiency disease. Bone Marrow Transplant. 2008; 41:119–126.
Article5. Warren EH, Fujii N, Akatsuka Y, Chaney CN, Mito JK, Loeb KR, Gooley TA, Brown ML, Koo KK, Rosinski KV, Ogawa S, Matsubara A, Appelbaum FR, Riddell SR. Therapy of relapsed leukemia after allogeneic hematopoietic cell transplantation with T cells specific for minor histocompatibility antigens. Blood. 2010; 115:3869–3878.
Article6. Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012; 12:443–458.
Article7. Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007; 7:340–352.
Article8. Washington K, Jagasia M. Pathology of graft-versus-host disease in the gastrointestinal tract. Hum Pathol. 2009; 40:909–917.
Article9. Hill GR, Ferrara JL. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood. 2000; 95:2754–2759.
Article10. Fukata M, Vamadevan AS, Abreu MT. Toll-like receptors (TLRs) and Nod-like receptors (NLRs) in inflammatory disorders. Semin Immunol. 2009; 21:242–253.
Article11. Heimesaat MM, Nogai A, Bereswill S, Plickert R, Fischer A, Loddenkemper C, Steinhoff U, Tchaptchet S, Thiel E, Freudenberg MA, Gobel UB, Uharek L. MyD88/TLR9 mediated immunopathology and gut microbiota dynamics in a novel murine model of intestinal graft-versus-host disease. Gut. 2010; 59:1079–1087.
Article12. Marcellus DC, Altomonte VL, Farmer ER, Horn TD, Freemer CS, Grant J, Vogelsang GB. Etretinate therapy for refractory sclerodermatous chronic graft-versus-host disease. Blood. 1999; 93:66–70.
Article13. Pai CC, Chen M, Mirsoian A, Grossenbacher SK, Tellez J, Ames E, Sun K, Jagdeo J, Blazar BR, Murphy WJ, Abedi M. Treatment of chronic graft-versus-host disease with bortezomib. Blood. 2014; 124:1677–1688.
Article14. Rozendaal L, Pals ST, Gleichmann E, Melief CJ. Persistence of allospecific helper T cells is required for maintaining autoantibody formation in lupus-like graft-versus-host disease. Clin Exp Immunol. 1990; 82:527–532.
Article15. Wakae T, Takatsuka H, Seto Y, Iwata N, Mori A, Okada M, Fujimori Y, Okamoto T, Kakishita E, Hara H. Similarity between hepatic graft-versus-host disease and primary biliary cirrhosis. Hematology. 2002; 7:305–310.
Article16. Shono Y, Shiratori S, Kosugi-Kanaya M, Ueha S, Sugita J, Shigematsu A, Kondo T, Hashimoto D, Fujimoto K, Endo T, Nishio M, Hashino S, Matsuno Y, Matsushima K, Tanaka J, Imamura M, Teshima T. Bone marrow graft-versus-host disease: evaluation of its clinical impact on disrupted hematopoiesis after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2014; 20:495–500.
Article17. Silva R, Morgado JM, Freitas A, Couceiro A, Orfao A, Regateiro F, Paiva A. Influence of pro- and anti-inflammatory cytokines in Th1 polarization after allogeneic stimulation. Int J Biomed Sci. 2005; 1:46–52.18. Biedermann BC, Sahner S, Gregor M, Tsakiris DA, Jeanneret C, Pober JS, Gratwohl A. Endothelial injury mediated by cytotoxic T lymphocytes and loss of microvessels in chronic graft versus host disease. Lancet. 2002; 359:2078–2083.
Article19. Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, Palmer J, Weisdorf D, Treister NS, Cheng GS, Kerr H, Stratton P, Duarte RF, McDonald GB, Inamoto Y, Vigorito A, Arai S, Datiles MB, Jacobsohn D, Heller T, Kitko CL, Mitchell SA, Martin PJ, Shulman H, Wu RS, Cutler CS, Vogelsang GB, Lee SJ, Pavletic SZ, Flowers ME. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 diagnosis and staging working group report. Biol Blood Marrow Transplant. 2015; 21:389–401.
Article20. Ferrara JL, Deeg HJ. Graft-versus-host disease. N Engl J Med. 1991; 324:667–674.
Article21. Reddy P. Pathophysiology of acute graft-versus-host disease. Hematol Oncol. 2003; 21:149–161.
Article22. Ju JM, Lee H, Oh K, Lee DS, Choi EY. Kinetics of IFN-gamma and IL-17 production by CD4 and CD8 T cells during acute graft-versus-host disease. Immune Netw. 2014; 14:89–99.
Article23. Ross WA, Ghosh S, Dekovich AA, Liu S, Ayers GD, Cleary KR, Lee JH, Couriel D. Endoscopic biopsy diagnosis of acute gastrointestinal graft-versus-host disease: rectosigmoid biopsies are more sensitive than upper gastrointestinal biopsies. Am J Gastroenterol. 2008; 103:982–989.
Article24. Mai V, Draganov PV. Recent advances and remaining gaps in our knowledge of associations between gut microbiota and human health. World J Gastroenterol. 2009; 15:81–85.
Article25. Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016; 14:e1002533.
Article26. Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977; 31:107–133.
Article27. Jenq RR, Ubeda C, Taur Y, Menezes CC, Khanin R, Dudakov JA, Liu C, West ML, Singer NV, Equinda MJ, Gobourne A, Lipuma L, Young LF, Smith OM, Ghosh A, Hanash AM, Goldberg JD, Aoyama K, Blazar BR, Pamer EG, van den Brink MR. Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J Exp Med. 2012; 209:903–911.
Article28. Eriguchi Y, Takashima S, Oka H, Shimoji S, Nakamura K, Uryu H, Shimoda S, Iwasaki H, Shimono N, Ayabe T, Akashi K, Teshima T. Graft-versus-host disease disrupts intestinal microbial ecology by inhibiting Paneth cell production of alpha-defensins. Blood. 2012; 120:223–231.
Article29. Beelen DW, Elmaagacli A, Muller KD, Hirche H, Schaefer UW. Influence of intestinal bacterial decontamination using metronidazole and ciprofloxacin or ciprofloxacin alone on the development of acute graft-versus-host disease after marrow transplantation in patients with hematologic malignancies: final results and long-term follow-up of an open-label prospective randomized trial. Blood. 1999; 93:3267–3275.
Article30. Murphy S, Nguyen VH. Role of gut microbiota in graft-versus-host disease. Leuk Lymphoma. 2011; 52:1844–1856.
Article31. Schwab L, Goroncy L, Palaniyandi S, Gautam S, Triantafyllopoulou A, Mocsai A, Reichardt W, Karlsson FJ, Radhakrishnan SV, Hanke K, Schmitt-Graeff A, Freudenberg M, von Loewenich FD, Wolf P, Leonhardt F, Baxan N, Pfeifer D, Schmah O, Schonle A, Martin SF, Mertelsmann R, Duyster J, Finke J, Prinz M, Henneke P, Hacker H, Hildebrandt GC, Hacker G, Zeiser R. Neutrophil granulocytes recruited upon translocation of intestinal bacteria enhance graft-versus-host disease via tissue damage. Nat Med. 2014; 20:648–654.
Article32. Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997; 90:3204–3213.
Article33. Cooke KR, Gerbitz A, Crawford JM, Teshima T, Hill GR, Tesolin A, Rossignol DP, Ferrara JL. LPS antagonism reduces graft-versus-host disease and preserves graft-versus-leukemia activity after experimental bone marrow transplantation. J Clin Invest. 2001; 107:1581–1589.
Article34. Holler E. Cytokines, viruses, and graft-versus-host disease. Curr Opin Hematol. 2002; 9:479–484.
Article35. Brennan TV, Lin L, Huang X, Cardona DM, Li Z, Dredge K, Chao NJ, Yang Y. Heparan sulfate, an endogenous TLR4 agonist, promotes acute GVHD after allogeneic stem cell transplantation. Blood. 2012; 120:2899–2908.
Article36. Imado T, Iwasaki T, Kitano S, Satake A, Kuroiwa T, Tsunemi S, Sano H. The protective role of host Toll-like receptor-4 in acute graft-versus-host disease. Transplantation. 2010; 90:1063–1070.
Article37. Li H, Matte-Martone C, Tan HS, Venkatesan S, McNiff J, Demetris AJ, Jain D, Lakkis F, Rothstein D, Shlomchik WD. Graft-versus-host disease is independent of innate signaling pathways triggered by pathogens in host hematopoietic cells. J Immunol. 2011; 186:230–241.
Article38. Calcaterra C, Sfondrini L, Rossini A, Sommariva M, Rumio C, Menard S, Balsari A. Critical role of TLR9 in acute graft-versus-host disease. J Immunol. 2008; 181:6132–6139.
Article39. Taylor PA, Ehrhardt MJ, Lees CJ, Panoskaltsis-Mortari A, Krieg AM, Sharpe AH, Murphy WJ, Serody JS, Hemmi H, Akira S, Levy RB, Blazar BR. TLR agonists regulate alloresponses and uncover a critical role for donor APCs in allogeneic bone marrow rejection. Blood. 2008; 112:3508–3516.
Article40. Spranger S, Javorovic M, Burdek M, Wilde S, Mosetter B, Tippmer S, Bigalke I, Geiger C, Schendel DJ, Frankenberger B. Generation of Th1-polarizing dendritic cells using the TLR7/8 agonist CL075. J Immunol. 2010; 185:738–747.
Article41. Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K, Akira S. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002; 3:196–200.
Article42. Jasperson LK, Bucher C, Panoskaltsis-Mortari A, Mellor AL, Munn DH, Blazar BR. Inducing the tryptophan catabolic pathway, indoleamine 2,3-dioxygenase (IDO), for suppression of graft-versus-host disease (GVHD) lethality. Blood. 2009; 114:5062–5070.
Article43. Lee WS, Kim JY, Won HJ, Lee SM, Suh YS, Joo YD, Lee JY, Jang WH, Kang SW, Kang MS, Park SG, Choi IW, Choi I, Seo SK. Effect of upregulated TLR2 expression from G-CSF-mobilized donor grafts on acute graft-versus-host disease. Int Immunopharmacol. 2015; 29:488–493.
Article44. Sivula J, Cordova ZM, Tuimala J, Jaatinen T, Partanen J, Volin L, Turpeinen H. Toll-like receptor gene polymorphisms confer susceptibility to graft-versus-host disease in allogenic hematopoietic stem cell transplantation. Scand J Immunol. 2012; 76:336–341.
Article45. Xiao HW, Luo Y, Lai XY, Shi JM, Tan YM, He JS, Xie WZ, Zheng WY, Ye XJ, Yu XH, Cai Z, Lin MF, Huang H. Donor TLR9 gene tagSNPs influence susceptibility to aGVHD and CMV reactivation in the allo-HSCT setting without polymorphisms in the TLR4 and NOD2 genes. Bone Marrow Transplant. 2014; 49:241–247.
Article46. Lorenz E, Schwartz DA, Martin PJ, Gooley T, Lin MT, Chien JW, Hansen JA, Clark JG. Association of TLR4 mutations and the risk for acute GVHD after HLA-matched-sibling hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2001; 7:384–387.
Article47. Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004; 4:499–511.
Article48. Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003; 21:335–376.
Article49. Lim JY, Lee YK, Lee SE, Ju JM, Park G, Choi EY, Min CK. Attenuation of hepatic graft-versus-host disease in allogeneic recipients of MyD88-deficient donor bone marrow. Immune Netw. 2015; 15:125–134.
Article50. Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003; 301:640–643.
Article51. Lim JY, Lee YK, Lee SE, Ju JM, Eom KS, Kim YJ, Chung NG, Jeong DC, Park G, Choi EY, Min CK. MyD88 in donor bone marrow cells is critical for protection from acute intestinal graft-vs.-host disease. Mucosal Immunol. 2016; 9:730–743.
Article52. Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012; 12:253–268.
Article53. Crook KR, Liu P. Role of myeloid-derived suppressor cells in autoimmune disease. World J Immunol. 2014; 4:26–33.
Article54. Smith AR, Reynolds JM. Editorial: the contribution of myeloid-derived suppression to inflammatory disease. J Leukoc Biol. 2014; 96:361–364.
Article55. Chandra D, Jahangir A, Quispe-Tintaya W, Einstein MH, Gravekamp C. Myeloid-derived suppressor cells have a central role in attenuated Listeria monocytogenes-based immunotherapy against metastatic breast cancer in young and old mice. Br J Cancer. 2013; 108:2281–2290.
Article56. Schmid M, Zimara N, Wege AK, Ritter U. Myeloid-derived suppressor cell functionality and interaction with Leishmania major parasites differ in C57BL/6 and BALB/c mice. Eur J Immunol. 2014; 44:3295–3306.
Article57. Terrazas LI, Walsh KL, Piskorska D, McGuire E, Harn DA Jr. The schistosome oligosaccharide lacto-N-neotetraose expands Gr1(+) cells that secrete anti-inflammatory cytokines and inhibit proliferation of naive CD4(+) cells: a potential mechanism for immune polarization in helminth infections. J Immunol. 2001; 167:5294–5303.
Article58. Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009; 9:162–174.
Article59. Koehn BH, Apostolova P, Haverkamp JM, Miller JS, McCullar V, Tolar J, Munn DH, Murphy WJ, Brickey WJ, Serody JS, Gabrilovich DI, Bronte V, Murray PJ, Ting JP, Zeiser R, Blazar BR. GVHD-associated, inflammasome-mediated loss of function in adoptively transferred myeloid-derived suppressor cells. Blood. 2015; 126:1621–1628.
Article60. Messmann JJ, Reisser T, Leithauser F, Lutz MB, Debatin KM, Strauss G. In vitro-generated MDSCs prevent murine GVHD by inducing type 2 T cells without disabling antitumor cytotoxicity. Blood. 2015; 126:1138–1148.
Article61. Kusmartsev S, Eruslanov E, Kubler H, Tseng T, Sakai Y, Su Z, Kaliberov S, Heiser A, Rosser C, Dahm P, Siemann D, Vieweg J. Oxidative stress regulates expression of VEGFR1 in myeloid cells: link to tumor-induced immune suppression in renal cell carcinoma. J Immunol. 2008; 181:346–353.
Article62. Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, Golshayan A, Rayman PA, Wood L, Garcia J, Dreicer R, Bukowski R, Finke JH. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009; 15:2148–2157.
Article63. De SC, Serafini P, Marigo I, Dolcetti L, Bolla M, Del SP, Melani C, Guiducci C, Colombo MP, Iezzi M, Musiani P, Zanovello P, Bronte V. Nitroaspirin corrects immune dysfunction in tumor-bearing hosts and promotes tumor eradication by cancer vaccination. Proc Natl Acad Sci U S A. 2005; 102:4185–4190.
Article64. Veltman JD, Lambers ME, van NM, Hendriks RW, Hoogsteden HC, Aerts JG, Hegmans JP. COX-2 inhibition improves immunotherapy and is associated with decreased numbers of myeloid-derived suppressor cells in mesothelioma. Celecoxib influences MDSC function. BMC Cancer. 2010; 10:464.
Article65. Wu H, Tao N, Liu X, Li X, Tang J, Ma C, Xu X, Shao H, Hou B, Wang H, Qin Z. Polysaccharide from Lentinus edodes inhibits the immunosuppressive function of myeloid-derived suppressor cells. PLoS One. 2012; 7:e51751.
Article66. Roth F, De La Fuente AC, Vella JL, Zoso A, Inverardi L, Serafini P. Aptamer-mediated blockade of IL4Ralpha triggers apoptosis of MDSCs and limits tumor progression. Cancer Res. 2012; 72:1373–1383.
Article67. Sawanobori Y, Ueha S, Kurachi M, Shimaoka T, Talmadge JE, Abe J, Shono Y, Kitabatake M, Kakimi K, Mukaida N, Matsushima K. Chemokine-mediated rapid turnover of myeloid-derived suppressor cells in tumor-bearing mice. Blood. 2008; 111:5457–5466.
Article68. Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007; 67:4507–4513.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Innate immunity and transplantation tolerance: the potential role of TLRs/NLRs in GVHD
- Regulation of Innate Immunity via MHC Class II-mediated Signaling; Non-classical Role of MHC Class II in Innate Immunity
- The Role of Toll-Like Receptor in Organ Transplantation
- Clinical Significance of Toll-Like Receptor and Toll-Like Receptor Blocker
- The Gate Way of Communication between Microorganism and Human Body: Toll-like Receptors