Korean J Hematol.
2011 Jun;46(2):69-79. 10.5045/kjh.2011.46.2.69.
Innate immunity and transplantation tolerance: the potential role of TLRs/NLRs in GVHD
- Affiliations
-
- 1Department of Medical Life Science Research, The Catholic University of Korea, School of Medicine, Seoul, Korea. oshin@catholic.ac.kr
- 2Division of Infectious Diseases, Massachusetts General Hospital, Boston, MA, USA.
- 3Department of Pediatrics, Harvard Medical School, Boston, MA, USA.
- KMID: 1782770
- DOI: http://doi.org/10.5045/kjh.2011.46.2.69
Abstract
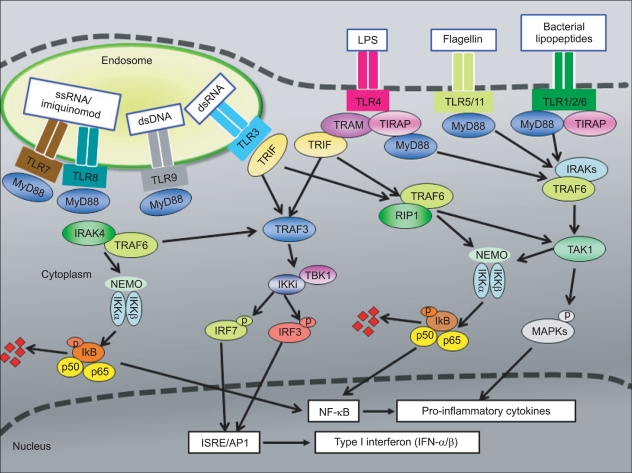
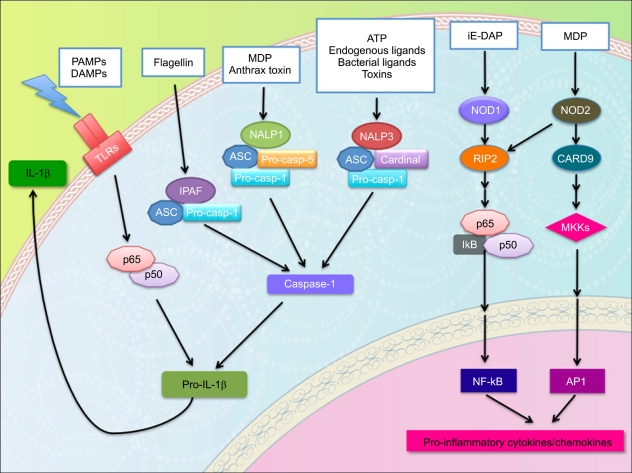
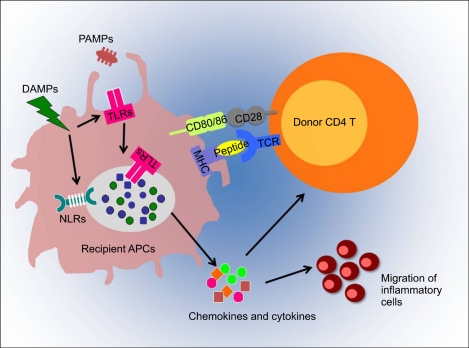
- Graft-versus-host disease (GVHD) is a serious complication of allogeneic hematopoietic cell transplantation (HCT) and this occurs as donor T lymphocytes, activated by recipient antigen presenting cells (APC), attack the host tissues or organs. This APC activation is a crucial initial step of influencing the outcome of GVHD and is mediated by innate immune signaling. Toll-like receptors (TLRs) and nucleotide binding oligomerization domain (NOD)-like receptors (NLRs) are important components of innate immunity; both families of receptors are known for sensing various microbial ligands or danger signals. Signaling through TLRs/NLRs regulate activities of APCs, through phagocytosis, cytokine and chemokine release, delivery of APCs from peripheral tissues to draining lymph nodes, and antigen presentation. Several TLRs/NLRs have been identified and their ligands and signaling pathways have been described. Recent findings suggest a significant association of TLR/NLR polymorphisms with the increased risk for severe GVHD. Therefore, these TLR/NLR pathways likely contributing to immune response for GVHD may serve as novel therapeutic targets to facilitate allograft tolerance. This review summarizes the role of TLRs/NLRs innate immune receptors and signaling in GVHD pathophysiology.
Keyword
MeSH Terms
Figure
Reference
-
1. Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010; 11:373–384. PMID: 20404851.
Article3. Matzinger P. The danger model: a renewed sense of self. Science. 2002; 296:301–305. PMID: 11951032.
Article4. Zeiser R, Penack O, Holler E, Idzko M. Danger signals activating innate immunity in graft-versus-host disease. J Mol Med. 2011; [Epub ahead of print].
Article5. Watson MJ, Ke B, Shen XD, et al. Intestinal ischemia/reperfusion injury triggers activation of innate toll-like receptor 4 and adaptive chemokine programs. Transplant Proc. 2008; 40:3339–3341. PMID: 19100385.
Article6. Landfried K, Bataille F, Rogler G, et al. Recipient NOD2/CARD15 status affects cellular infiltrates in human intestinal graft-versus-host disease. Clin Exp Immunol. 2010; 159:87–92. PMID: 19912254.
Article7. Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996; 86:973–983. PMID: 8808632.8. Takeda K, Takeuchi O, Akira S. Recognition of lipopeptides by Toll-like receptors. J Endotoxin Res. 2002; 8:459–463. PMID: 12697090.
Article9. Alexopoulou L, Thomas V, Schnare M, et al. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nat Med. 2002; 8:878–884. PMID: 12091878.
Article10. Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001; 413:732–738. PMID: 11607032.11. Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998; 282:2085–2088. PMID: 9851930.12. Brodsky I, Medzhitov R. Two modes of ligand recognition by TLRs. Cell. 2007; 130:979–981. PMID: 17889640.
Article13. Kim HM, Park BS, Kim JI, et al. Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell. 2007; 130:906–917. PMID: 17803912.
Article14. Hayashi F, Smith KD, Ozinsky A, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001; 410:1099–1103. PMID: 11323673.
Article15. Heil F, Hemmi H, Hochrein H, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004; 303:1526–1529. PMID: 14976262.
Article17. Hemmi H, Takeuchi O, Kawai T, et al. A toll-like receptor recognizes bacterial DNA. Nature. 2000; 408:740–745. PMID: 11130078.
Article18. Yarovinsky F, Zhang D, Andersen JF, et al. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science. 2005; 308:1626–1629. PMID: 15860593.
Article19. Zhang D, Zhang G, Hayden MS, et al. A toll-like receptor that prevents infection by uropathogenic bacteria. Science. 2004; 303:1522–1526. PMID: 15001781.
Article20. Hacker H, Redecke V, Blagoev B, et al. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature. 2006; 439:204–207. PMID: 16306937.
Article21. Oganesyan G, Saha SK, Guo B, et al. Critical role of TRAF3 in the Toll-like receptor-dependent and -independent antiviral response. Nature. 2006; 439:208–211. PMID: 16306936.
Article22. Jiang Z, Mak TW, Sen G, Li X. Toll-like receptor 3-mediated activation of NF-kappaB and IRF3 diverges at Toll-IL-1 receptor domain-containing adapter inducing IFN-beta. Proc Natl Acad Sci U S A. 2004; 101:3533–3538. PMID: 14982987.23. Sato S, Sugiyama M, Yamamoto M, et al. Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta (TRIF) associates with TNF receptor-associated factor 6 and TANK-binding kinase 1, and activates two distinct transcription factors, NF-kappa B and IFN-regulatory factor-3, in the Toll-like receptor signaling. J Immunol. 2003; 171:4304–4310. PMID: 14530355.24. Hacker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci STKE. 2006; 2006:re13. PMID: 17047224.
Article25. Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004; 5:987–995. PMID: 15454922.
Article26. Ellett JD, Evans ZP, Atkinson C, Schmidt MG, Schnellmann RG, Chavin KD. Toll-like receptor 4 is a key mediator of murine steatotic liver warm ischemia/reperfusion injury. Liver Transpl. 2009; 15:1101–1109. PMID: 19718644.
Article27. Hui W, Jinxiang Z, Heshui W, Zhuoya L, Qichang Z. Bone marrow and non-bone marrow TLR4 regulates hepatic ischemia/reperfusion injury. Biochem Biophys Res Commun. 2009; 389:328–332. PMID: 19723506.
Article28. Kaczorowski DJ, Nakao A, Vallabhaneni R, et al. Mechanisms of Toll-like receptor 4 (TLR4)-mediated inflammation after cold ischemia/reperfusion in the heart. Transplantation. 2009; 87:1455–1463. PMID: 19461481.
Article29. Zhai Y, Qiao B, Shen XD, et al. Evidence for the pivotal role of endogenous toll-like receptor 4 ligands in liver ischemia and reperfusion injury. Transplantation. 2008; 85:1016–1022. PMID: 18408583.
Article30. Zhai Y, Shen XD, O'Connell R, et al. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J Immunol. 2004; 173:7115–7119. PMID: 15585830.
Article31. Cooke KR, Gerbitz A, Crawford JM, et al. LPS antagonism reduces graft-versus-host disease and preserves graft-versus-leukemia activity after experimental bone marrow transplantation. J Clin Invest. 2001; 107:1581–1589. PMID: 11413166.
Article32. Goldstein DR, Tesar BM, Akira S, Lakkis FG. Critical role of the Toll-like receptor signal adaptor protein MyD88 in acute allograft rejection. J Clin Invest. 2003; 111:1571–1578. PMID: 12750407.
Article33. Tesar BM, Zhang J, Li Q, Goldstein DR. TH1 immune responses to fully MHC mismatched allografts are diminished in the absence of MyD88, a toll-like receptor signal adaptor protein. Am J Transplant. 2004; 4:1429–1439. PMID: 15307830.
Article34. Lorenz E, Schwartz DA, Martin PJ, et al. Association of TLR4 mutations and the risk for acute GVHD after HLA-matched-sibling hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2001; 7:384–387. PMID: 11529488.
Article35. Elmaagacli AH, Koldehoff M, Hindahl H, et al. Mutations in innate immune system NOD2/CARD 15 and TLR-4 (Thr399Ile) genes influence the risk for severe acute graft-versus-host disease in patients who underwent an allogeneic transplantation. Transplantation. 2006; 81:247–254. PMID: 16436969.
Article36. Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004; 118:229–241. PMID: 15260992.
Article37. Ito T, Amakawa R, Kaisho T, et al. Interferon-alpha and interleukin-12 are induced differentially by Toll-like receptor 7 ligands in human blood dendritic cell subsets. J Exp Med. 2002; 195:1507–1512. PMID: 12045249.38. Jasperson LK, Bucher C, Panoskaltsis-Mortari A, et al. Indoleamine 2,3-dioxygenase is a critical regulator of acute graft-versus-host disease lethality. Blood. 2008; 111:3257–3265. PMID: 18077788.
Article39. Jasperson LK, Bucher C, Panoskaltsis-Mortari A, Mellor AL, Munn DH, Blazar BR. Inducing the tryptophan catabolic pathway, indoleamine 2,3-dioxygenase (IDO), for suppression of graft-versus-host disease (GVHD) lethality. Blood. 2009; 114:5062–5070. PMID: 19828695.
Article40. Taylor PA, Ehrhardt MJ, Lees CJ, et al. TLR agonists regulate alloresponses and uncover a critical role for donor APCs in allogeneic bone marrow rejection. Blood. 2008; 112:3508–3516. PMID: 18614760.
Article41. McKay D, Shigeoka A, Rubinstein M, Surh C, Sprent J. Simultaneous deletion of MyD88 and Trif delays major histocompatibility and minor antigen mismatch allograft rejection. Eur J Immunol. 2006; 36:1994–2002. PMID: 16874736.
Article42. Kulkarni R, Behboudi S, Sharif S. Insights into the role of Toll-like receptors in modulation of T cell responses. Cell Tissue Res. 2011; 343:141–152. PMID: 20680345.
Article43. Gelman AE, Zhang J, Choi Y, Turka LA. Toll-like receptor ligands directly promote activated CD4+ T cell survival. J Immunol. 2004; 172:6065–6073. PMID: 15128790.44. Caron G, Duluc D, Fremaux I, et al. Direct stimulation of human T cells via TLR5 and TLR7/8: flagellin and R-848 up-regulate proliferation and IFN-gamma production by memory CD4+ T cells. J Immunol. 2005; 175:1551–1557. PMID: 16034093.45. Komai-Koma M, Jones L, Ogg GS, Xu D, Liew FY. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc Natl Acad Sci U S A. 2004; 101:3029–3034. PMID: 14981245.
Article46. Chen L, Wang T, Zhou P, et al. TLR engagement prevents transplantation tolerance. Am J Transplant. 2006; 6:2282–2291. PMID: 16970798.
Article47. Heimesaat MM, Nogai A, Bereswill S, et al. MyD88/TLR9 mediated immunopathology and gut microbiota dynamics in a novel murine model of intestinal graft-versus-host disease. Gut. 2010; 59:1079–1087. PMID: 20639251.
Article48. Holler E, Landfried K, Meier J, Hausmann M, Rogler G. The role of bacteria and pattern recognition receptors in GVHD. Int J Inflam. 2010; 2010:814326. PMID: 21188220.
Article49. Gerbitz A, Schultz M, Wilke A, et al. Probiotic effects on experimental graft-versus-host disease: let them eat yogurt. Blood. 2004; 103:4365–4367. PMID: 14962899.
Article50. van Bekkum DW, Roodenburg J, Heidt PJ, van der Waaij D. Mitigation of secondary disease of allogeneic mouse radiation chimeras by modification of the intestinal microflora. J Natl Cancer Inst. 1974; 52:401–404. PMID: 4150164.51. Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002; 10:417–426. PMID: 12191486.52. Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004; 20:319–325. PMID: 15030775.53. Tschopp J, Martinon F, Burns K. NALPs: a novel protein family involved in inflammation. Nat Rev Mol Cell Biol. 2003; 4:95–104. PMID: 12563287.
Article54. Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009; 27:229–265. PMID: 19302040.
Article55. Martinon F, Tschopp J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell. 2004; 117:561–574. PMID: 15163405.56. Latz E. The inflammasomes: mechanisms of activation and function. Curr Opin Immunol. 2010; 22:28–33. PMID: 20060699.
Article57. Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001; 411:599–603. PMID: 11385576.
Article58. Ogura Y, Bonen DK, Inohara N, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001; 411:603–606. PMID: 11385577.
Article59. Chamaillard M, Hashimoto M, Horie Y, et al. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat Immunol. 2003; 4:702–707. PMID: 12796777.
Article60. Girardin SE, Boneca IG, Viala J, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003; 278:8869–8872. PMID: 12527755.
Article61. Viala J, Chaput C, Boneca IG, et al. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat Immunol. 2004; 5:1166–1174. PMID: 15489856.
Article62. Kobayashi K, Inohara N, Hernandez LD, et al. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature. 2002; 416:194–199. PMID: 11894098.
Article63. Kufer TA, Banks DJ, Philpott DJ. Innate immune sensing of microbes by Nod proteins. Ann N Y Acad Sci. 2006; 1072:19–27. PMID: 17057187.
Article64. Kobayashi KS, Chamaillard M, Ogura Y, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005; 307:731–734. PMID: 15692051.
Article65. Ogura Y, Inohara N, Benito A, Chen FF, Yamaoka S, Nunez G. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem. 2001; 276:4812–4818. PMID: 11087742.66. Bruey JM, Bruey-Sedano N, Luciano F, et al. Bcl-2 and Bcl-XL regulate proinflammatory caspase-1 activation by interaction with NALP1. Cell. 2007; 129:45–56. PMID: 17418785.67. Lamkanfi M, Dixit VM. Inflammasomes: guardians of cytosolic sanctity. Immunol Rev. 2009; 227:95–105. PMID: 19120479.
Article68. Kanneganti TD, Body-Malapel M, Amer A, et al. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J Biol Chem. 2006; 281:36560–36568. PMID: 17008311.
Article69. Zheng Y, Lilo S, Brodsky IE, et al. A Yersinia effector with enhanced inhibitory activity on the NF-κB pathway activates the NLRP3/ASC/caspase-1 Inflammasome in macrophages. PLoS Pathog. 2011; 7:e1002026. PMID: 21533069.
Article70. Nour AM, Reichelt M, Ku CC, Ho MY, Heineman TC, Arvin AM. Varicella-zoster virus infection triggers formation of an interleukin-1{beta} (IL-1{beta})-processing inflammasome complex. J Biol Chem. 2011; 286:17921–17933. PMID: 21385879.71. Joly S, Sutterwala FS. Fungal pathogen recognition by the NLRP3 inflammasome. Virulence. 2010; 1:276–280. PMID: 21178453.
Article72. McNeela EA, Burke A, Neill DR, et al. Pneumolysin activates the NLRP3 inflammasome and promotes proinflammatory cytokines independently of TLR4. PLoS Pathog. 2010; 6:e1001191. PMID: 21085613.
Article73. Willingham SB, Bergstralh DT, O'Connor W, et al. Microbial pathogen-induced necrotic cell death mediated by the inflammasome components CIAS1/cryopyrin/NLRP3 and ASC. Cell Host Microbe. 2007; 2:147–159. PMID: 18005730.
Article74. Craven RR, Gao X, Allen IC, et al. Staphylococcus aureus alpha-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PLoS One. 2009; 4:e7446. PMID: 19826485.75. Duewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010; 464:1357–1361. PMID: 20428172.
Article76. Halle A, Hornung V, Petzold GC, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008; 9:857–865. PMID: 18604209.77. Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006; 440:237–241. PMID: 16407889.
Article78. Mariathasan S, Weiss DS, Newton K, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006; 440:228–232. PMID: 16407890.
Article79. Miao EA, Alpuche-Aranda CM, Dors M, et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol. 2006; 7:569–575. PMID: 16648853.80. Franchi L, Amer A, Body-Malapel M, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol. 2006; 7:576–582. PMID: 16648852.81. Amer A, Franchi L, Kanneganti TD, et al. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J Biol Chem. 2006; 281:35217–35223. PMID: 16984919.
Article82. Sun YH, Rolán HG, Tsolis RM. Injection of flagellin into the host cell cytosol by Salmonella enterica serotype Typhimurium. J Biol Chem. 2007; 282:33897–33901. PMID: 17911114.
Article83. Sutterwala FS, Flavell RA. NLRC4/IPAF: a CARD carrying member of the NLR family. Clin Immunol. 2009; 130:2–6. PMID: 18819842.
Article84. Sutterwala FS, Ogura Y, Szczepanik M, et al. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006; 24:317–327. PMID: 16546100.
Article85. Kawaguchi M, Takahashi M, Hata T, et al. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation. 2011; 123:594–604. PMID: 21282498.
Article86. Yajima N, Takahashi M, Morimoto H, et al. Critical role of bone marrow apoptosis-associated speck-like protein, an inflammasome adaptor molecule, in neointimal formation after vascular injury in mice. Circulation. 2008; 117:3079–3087. PMID: 18541743.
Article87. Zhu P, Duan L, Chen J, et al. Gene Silencing of NALP3 Protects Against Liver Ischemia-Reperfusion Injury in Mice. Hum Gene Ther. 2011; [Epub ahead of print].
Article88. Holler E, Rogler G, Brenmoehl J, et al. Prognostic significance of NOD2/CARD15 variants in HLA-identical sibling hematopoietic stem cell transplantation: effect on long-term outcome is confirmed in 2 independent cohorts and may be modulated by the type of gastrointestinal decontamination. Blood. 2006; 107:4189–4193. PMID: 16424393.
Article89. Holler E, Rogler G, Herfarth H, et al. Both donor and recipient NOD2/CARD15 mutations associate with transplant-related mortality and GvHD following allogeneic stem cell transplantation. Blood. 2004; 104:889–894. PMID: 15090455.
Article90. Hsu LC, Ali SR, McGillivray S, et al. A NOD2-NALP1 complex mediates caspase-1-dependent IL-1beta secretion in response to Bacillus anthracis infection and muramyl dipeptide. Proc Natl Acad Sci U S A. 2008; 105:7803–7808. PMID: 18511561.91. van der Velden WJ, Blijlevens NM, Maas FM, et al. NOD2 polymorphisms predict severe acute graft-versus-host and treatment-related mortality in T-cell-depleted haematopoietic stem cell transplantation. Bone Marrow Transplant. 2009; 44:243–248. PMID: 19219079.
Article92. Granell M, Urbano-Ispizua A, Pons A, et al. Common variants in NLRP2 and NLRP3 genes are strong prognostic factors for the outcome of HLA-identical sibling allogeneic stem cell transplantation. Blood. 2008; 112:4337–4342. PMID: 18772453.
Article93. Penack O, Smith OM, Cunningham-Bussel A, et al. NOD2 regulates hematopoietic cell function during graft-versus-host disease. J Exp Med. 2009; 206:2101–2110. PMID: 19737867.
Article94. Penack O, Holler E, van den Brink MR. Graft-versus-host disease: regulation by microbe-associated molecules and innate immune receptors. Blood. 2010; 115:1865–1872. PMID: 20042727.
Article95. Granell M, Urbano-Ispizua A, Arostegui JI, et al. Effect of NOD2/CARD15 variants in T-cell depleted allogeneic stem cell transplantation. Haematologica. 2006; 91:1372–1376. PMID: 17018387.96. Gruhn B, Intek J, Pfaffendorf N, et al. Polymorphism of interleukin-23 receptor gene but not of NOD2/CARD15 is associated with graft-versus-host disease after hematopoietic stem cell transplantation in children. Biol Blood Marrow Transplant. 2009; 15:1571–1577. PMID: 19896081.
Article97. Sairafi D, Uzunel M, Remberger M, Ringden O, Mattsson J. No impact of NOD2/CARD15 on outcome after SCT. Bone Marrow Transplant. 2008; 41:961–964. PMID: 18317454.
Article98. Mayor NP, Shaw BE, Madrigal JA, Marsh SG. No impact of NOD2/CARD15 on outcome after SCT: a reply. Bone Marrow Transplant. 2008; 42:837–838. PMID: 18695658.
Article99. Dickinson AM, Holler E. Polymorphisms of cytokine and innate immunity genes and GVHD. Best Pract Res Clin Haematol. 2008; 21:149–164. PMID: 18503983.
Article100. Cullup H, Dickinson AM, Cavet J, Jackson GH, Middleton PG. Polymorphisms of interleukin-1alpha constitute independent risk factors for chronic graft-versus-host disease after allogeneic bone marrow transplantation. Br J Haematol. 2003; 122:778–787. PMID: 12930389.101. Wilhelm K, Ganesan J, Muller T, et al. Graft-versus-host disease is enhanced by extracellular ATP activating P2X7R. Nat Med. 2010; 16:1434–1438. PMID: 21102458.
Article102. Shlomchik WD, Couzens MS, Tang CB, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999; 285:412–415. PMID: 10411505.
Article103. Aksentijevich I, Masters SL, Ferguson PJ, et al. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N Engl J Med. 2009; 360:2426–2437. PMID: 19494218.