Int J Stem Cells.
2015 May;8(1):79-89. 10.15283/ijsc.2015.8.1.79.
Epigenetic Alterations of IL-6/STAT3 Signaling by Placental Stem Cells Promote Hepatic Regeneration in a Rat Model with CCl4-induced Liver Injury
- Affiliations
-
- 1Department of Biomedical Science, CHA University, Seongnam, Korea. gjkim@cha.ac.kr
- 2Department of Nanobiomedical Science, Dankook University, Cheonan, Korea.
- 3Institute of Human Genetics, Department of Anatomy, Korea University College of Medicine, Seoul, Korea. parksh@korea.ac.kr
- KMID: 2380801
- DOI: http://doi.org/10.15283/ijsc.2015.8.1.79
Abstract
- BACKGROUND
Human chorionic plate-derived mesenchymal stem cells (CP-MSCs) isolated from the placenta have been reported to demonstrate therapeutic effects in animal models of liver injury; however, the underlying epigenetic mechanism of this effect has not been elucidated. Thus, we investigated whether CP-MSCs influence epigenetic processes during regeneration of the injured liver.
METHODS
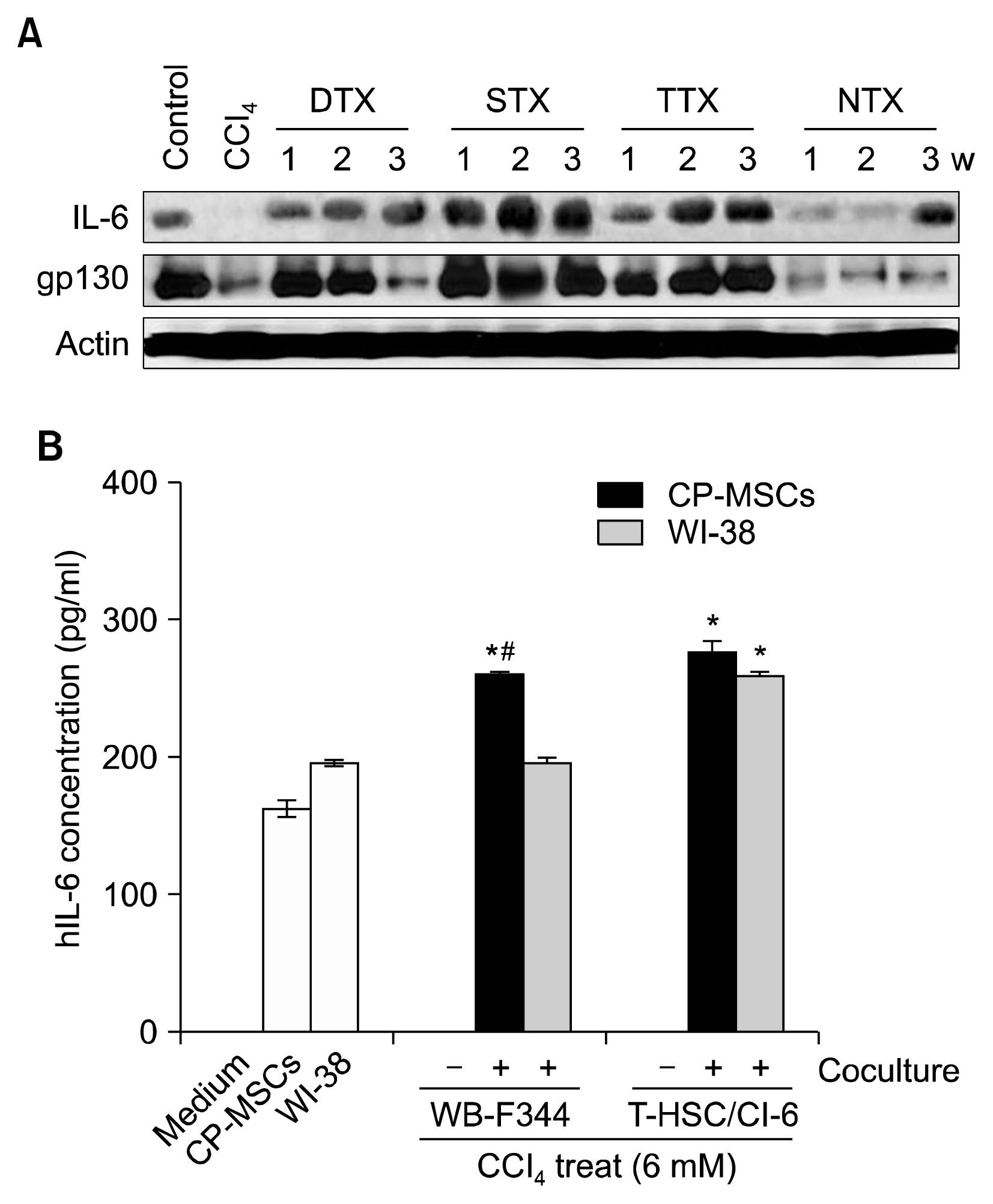
CP-MSCs were engrafted into a carbon tetrachloride (CCl4)-injured rat model through direct transplantation into the liver (DTX), intrasplenic transplantation (STX), and intravenous transplantation via the tail vein (TTX). Non-transplanted (NTX) rats were maintained as sham controls. Liver tissues were analyzed after transplantation using immunohistochemistry, western blot analysis, and quantitative methylation-specific polymerase chain reaction. Proliferation and human interleukin-6 (hIL-6) enzyme-linked immunosorbent assays were performed using CCl4-treated hepatic cells that were co-cultured with CP-MSCs.
RESULTS
The Ki67 labeling index, cell cyclins, albumin, IL-6, and gp130 levels were elevated in the CP-MSC transplantation groups. The concentration of hIL-6 in supernatants and the proliferation of CCl4-treated rat hepatic cells were enhanced by co-culturing with CP-MSCs (p<0.05), while the methylation of IL-6/IL-6R and STAT3 by CP-MSC transplantation decreased.
CONCLUSION
These results suggest that administration of CP-MSCs promotes IL-6/STAT3 signaling by decreasing the methylation of the IL-6/SATA3 promoters and thus inducing the proliferation of hepatic cells in a CCl4-injured liver rat model. These data advance our understanding of the therapeutic mechanisms in injured livers, and can facilitate the development of cell-based therapies using placenta-derived stem cells.
Keyword
MeSH Terms
-
Animals
Blotting, Western
Carbon Tetrachloride
Chorion
Cyclins
DNA Methylation
Enzyme-Linked Immunosorbent Assay
Epigenesis, Genetic
Epigenomics*
Hepatocytes
Humans
Immunohistochemistry
Interleukin-6
Liver Regeneration
Liver*
Mesenchymal Stromal Cells
Methylation
Models, Animal*
Placenta
Polymerase Chain Reaction
Rats
Regeneration*
Stem Cells*
Veins
Carbon Tetrachloride
Cyclins
Interleukin-6
Figure
Cited by 2 articles
-
Advanced Research on Stem Cell Therapy for Hepatic Diseases: Potential Implications of a Placenta-derived Mesenchymal Stem Cell-based Strategy
Gi Jin Kim
Hanyang Med Rev. 2015;35(4):207-214. doi: 10.7599/hmr.2015.35.4.207.Upregulation of C-Reactive Protein by Placenta-Derived Mesenchymal Stem Cells Promotes Angiogenesis in A Rat Model with Cirrhotic Liver
Ji Hye Jun, Jieun Jung, Jae Yeon Kim, Seong-Gyu Hwang, Si Hyun Bae, Gi Jin Kim
Int J Stem Cells. 2020;13(3):404-413. doi: 10.15283/ijsc20052.
Reference
-
References
1. Török NJ. Recent advances in the pathogenesis and diagnosis of liver fibrosis. J Gastroenterol. 2008; 43:315–321. DOI: 10.1007/s00535-008-2181-x. PMID: 18592147.
Article2. Lee DS, Gil WH, Lee HH, Lee KW, Lee SK, Kim SJ, Choi SH, Heo JS, Hyon WS, Kim GS, Paik SW, Koh KC, Joh JW. Factors affecting graft survival after living donor liver transplantation. Transplant Proc. 2004; 36:2255–2256. DOI: 10.1016/j.transproceed.2004.08.073. PMID: 15561210.
Article3. Horslen SP, Fox IJ. Hepatocyte transplantation. Transplantation. 2004; 77:1481–1486. DOI: 10.1097/01.TP.0000113809.53415.C2. PMID: 15239608.
Article4. Nussler A, Konig S, Ott M, Sokal E, Christ B, Thasler W, Brulport M, Gabelein G, Schormann W, Schulze M, Ellis E, Kraemer M, Nocken F, Fleig W, Manns M, Strom SC, Hengstler JG. Present status and perspectives of cell-based therapies for liver diseases. J Hepatol. 2006; 45:144–159. DOI: 10.1016/j.jhep.2006.04.002. PMID: 16730092.
Article5. Ren G, Chen X, Dong F, Li W, Ren X, Zhang Y, Shi Y. Concise review: mesenchymal stem cells and translational medicine: emerging issues. Stem Cells Transl Med. 2012; 1:51–58. DOI: 10.5966/sctm.2011-0019. PMID: 23197640. PMCID: 3727691.
Article6. Cai YF, Zhen ZJ, Min J, Fang TL, Chu ZH, Chen JS. Selection, proliferation and differentiation of bone marrow-derived liver stem cells with a culture system containing cholestatic serum in vitro. World J Gastroenterol. 2004; 10:3308–3312. PMID: 15484306.
Article7. Xu YQ, Liu ZC. Therapeutic potential of adult bone marrow stem cells in liver disease and delivery approaches. Stem Cell Rev. 2008; 4:101–112. DOI: 10.1007/s12015-008-9019-z. PMID: 18481229.
Article8. Rao MS, Mattson MP. Stem cells and aging: expanding the possibilities. Mech Ageing Dev. 2001; 122:713–734. DOI: 10.1016/S0047-6374(01)00224-X. PMID: 11322994.
Article9. Chakraborty A, Lazova R, Davies S, Bäckvall H, Ponten F, Brash D, Pawelek J. Donor DNA in a renal cell carcinoma metastasis from a bone marrow transplant recipient. Bone Marrow Transplant. 2004; 34:183–186. DOI: 10.1038/sj.bmt.1704547. PMID: 15195072.
Article10. Yilmaz Y, Lazova R, Qumsiyeh M, Cooper D, Pawelek J. Donor Y chromosome in renal carcinoma cells of a female BMT recipient: visualization of putative BMT-tumor hybrids by FISH. Bone Marrow Transplant. 2005; 35:1021–1024. DOI: 10.1038/sj.bmt.1704939. PMID: 15778726.
Article11. Houlihan DD, Newsome PN. Critical review of clinical trials of bone marrow stem cells in liver disease. Gastroenterology. 2008; 135:438–450. DOI: 10.1053/j.gastro.2008.05.040. PMID: 18585384.
Article12. Barlow S, Brooke G, Chatterjee K, Price G, Pelekanos R, Rossetti T, Doody M, Venter D, Pain S, Gilshenan K, Atkinson K. Comparison of human placenta- and bone marrow-derived multipotent mesenchymal stem cells. Stem Cells Dev. 2008; 17:1095–1107. PMID: 19006451.
Article13. Parolini O, Alviano F, Bagnara GP, Bilic G, Bühring HJ, Evangelista M, Hennerbichler S, Liu B, Magatti M, Mao N, Miki T, Marongiu F, Nakajima H, Nikaido T, Portmann-Lanz CB, Sankar V, Soncini M, Stadler G, Surbek D, Takahashi TA, Redl H, Sakuragawa N, Wolbank S, Zeisberger S, Zisch A, Strom SC. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells. 2008; 26:300–311. DOI: 10.1634/stemcells.2007-0594.
Article14. Jones BJ, Brooke G, Atkinson K, McTaggart SJ. Immuno-suppression by placental indoleamine 2,3-dioxygenase: a role for mesenchymal stem cells. Placenta. 2007; 28:1174–1181. DOI: 10.1016/j.placenta.2007.07.001. PMID: 17714779.
Article15. Kim MJ, Shin KS, Jeon JH, Lee DR, Shim SH, Kim JK, Cha DH, Yoon TK, Kim GJ. Human chorionic- plate-derived mesenchymal stem cells and Wharton’s jelly-derived mesenchymal stem cells: a comparative analysis of their potential as placenta-derived stem cells. Cell Tissue Res. 2011; 346:53–64. PMID: 21987220.
Article16. Lee MJ, Jung J, Na KH, Moon JS, Lee HJ, Kim JH, Kim GI, Kwon SW, Hwang SG, Kim GJ. Anti-fibrotic effect of chorionic plate-derived mesenchymal stem cells isolated from human placenta in a rat model of CCl4-injured liver: potential application to the treatment of hepatic diseases. J Cell Biochem. 2010; 111:1453–1463. PMID: 20830742.
Article17. Lee JM, Jung J, Lee HJ, Jeong SJ, Cho KJ, Hwang SG, Kim GJ. Comparison of immunomodulatory effects of placenta mesenchymal stem cells with bone marrow and adipose mesenchymal stem cells. Int Immunopharmacol. 2012; 13:219–224. PMID: 22487126.
Article18. Tsai PC, Fu TW, Chen YM, Ko TL, Chen TH, Shih YH, Hung SC, Fu YS. The therapeutic potential of human umbilical mesenchymal stem cells from Wharton’s jelly in the treatment of rat liver fibrosis. Liver Transpl. 2009; 15:484–495. PMID: 19399744.
Article19. Nakamura T, Torimura T, Sakamoto M, Hashimoto O, Taniguchi E, Inoue K, Sakata R, Kumashiro R, Murohara T, Ueno T, Sata M. Significance and therapeutic potential of endothelial progenitor cell transplantation in a cirrhotic liver rat model. Gastroenterology. 2007; 133:91–107. e101DOI: 10.1053/j.gastro.2007.03.110. PMID: 17631135.
Article20. Streetz KL, Luedde T, Manns MP, Trautwein C. Interleukin 6 and liver regeneration. Gut. 2000; 47:309–312. DOI: 10.1136/gut.47.2.309. PMID: 10896929. PMCID: 1727989.
Article21. Tiberio GA, Tiberio L, Benetti A, Cervi E, Montani N, Dreano M, Garotta G, Cerea K, Steimberg N, Pandolfo G, Ferrari-Bravo A, Mazzoleni G, Giulini SM, Schiaffonati L. IL-6 Promotes compensatory liver regeneration in cirrhotic rat after partial hepatectomy. Cytokine. 2008; 42:372–378. DOI: 10.1016/j.cyto.2008.03.012. PMID: 18455423.
Article22. Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996; 274:1379–1383. DOI: 10.1126/science.274.5291.1379. PMID: 8910279.
Article23. Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004; 5:836–847. DOI: 10.1038/nrm1489. PMID: 15459664.
Article24. Tron K, Samoylenko A, Musikowski G, Kobe F, Immenschuh S, Schaper F, Ramadori G, Kietzmann T. Regulation of rat heme oxygenase-1 expression by interleukin-6 via the Jak/STAT pathway in hepatocytes. J Hepatol. 2006; 45:72–80. DOI: 10.1016/j.jhep.2005.12.019. PMID: 16510205.
Article25. Song L, Webb NE, Song Y, Tuan RS. Identification and functional analysis of candidate genes regulating mesenchymal stem cell self-renewal and multipotency. Stem Cells. 2006; 24:1707–1718. DOI: 10.1634/stemcells.2005-0604. PMID: 16574750.
Article26. Menicanin D, Bartold PM, Zannettino AC, Gronthos S. Genomic profiling of mesenchymal stem cells. Stem Cell Rev. 2009; 5:36–50. DOI: 10.1007/s12015-009-9056-2. PMID: 19224407.
Article27. Reister S, Kordes C, Sawitza I, Häussinger D. The epigenetic regulation of stem cell factors in hepatic stellate cells. Stem Cells Dev. 2011; 20:1687–1699. DOI: 10.1089/scd.2010.0418. PMID: 21219128.
Article28. Robinson CM, Watson CJ, Baugh JA. Epigenetics within the matrix: a neo-regulator of fibrotic disease. Epigenetics. 2012; 7:987–993. DOI: 10.4161/epi.21567. PMID: 22894907. PMCID: 3515019.29. Jung J, Choi JH, Lee Y, Park JW, Oh IH, Hwang SG, Kim KS, Kim GJ. Human placenta-derived mesenchymal stem cells promote hepatic regeneration in CCl4-injured rat liver model via increased autophagic mechanism. Stem Cells. 2013; 31:1584–1596. DOI: 10.1002/stem.1396. PMID: 23592412.
Article30. Wuestefeld T, Klein C, Streetz KL, Betz U, Lauber J, Buer J, Manns MP, Müller W, Trautwein C. Interleukin-6/glyco-protein 130-dependent pathways are protective during liver regeneration. J Biol Chem. 2003; 278:11281–11288. DOI: 10.1074/jbc.M208470200. PMID: 12509437.
Article31. Sen GL, Reuter JA, Webster DE, Zhu L, Khavari PA. DNMT1 maintains progenitor function in self-renewing somatic tissue. Nature. 2010; 463:563–567. DOI: 10.1038/nature08683. PMID: 20081831. PMCID: 3050546.
Article32. Woo DH, Kim SK, Lim HJ, Heo J, Park HS, Kang GY, Kim SE, You HJ, Hoeppner DJ, Kim Y, Kwon H, Choi TH, Lee JH, Hong SH, Song KW, Ahn EK, Chenoweth JG, Tesar PJ, McKay RD, Kim JH. Direct and indirect contribution of human embryonic stem cell-derived hepatocyte-like cells to liver repair in mice. Gastroenterology. 2012; 142:602–611. DOI: 10.1053/j.gastro.2011.11.030.
Article33. Russo FP, Parola M. Stem cells in liver failure. Best Pract Res Clin Gastroenterol. 2012; 26:35–45. DOI: 10.1016/j.bpg.2012.01.001. PMID: 22482524.
Article34. Kuo TK, Hung SP, Chuang CH, Chen CT, Shih YR, Fang SC, Yang VW, Lee OK. Stem cell therapy for liver disease: parameters governing the success of using bone marrow mesenchymal stem cells. Gastroenterology. 2008; 134:2111– 2121. 2121 e2111–2113. DOI: 10.1053/j.gastro.2008.03.015. PMID: 18455168. PMCID: 3086672.
Article35. Lee Y, Jung J, Cho KJ, Lee SK, Park JW, Oh IH, Kim GJ. Increased SCF/c-kit by hypoxia promotes autophagy of human placental chorionic plate-derived mesenchymal stem cells via regulating the phosphorylation of mTOR. J Cell Biochem. 2013; 114:79–88. DOI: 10.1002/jcb.24303.
Article36. Jung J, Na KH, Lee MJ, Moon J, Kim GI, Jang JJ, Hwang SG, Kim GJ. Efficacy of chorionic plate-derived mesenchymal stem cells isolated from placenta in CCl4-injured rat model depends on transplantation routes. Tissue Engineering and Regenerative Medicine. 2013; 10:10–17. DOI: 10.1007/s13770-013-0364-x.
Article37. Tilg H, Wilmer A, Vogel W, Herold M, Nölchen B, Judmaier G, Huber C. Serum levels of cytokines in chronic liver diseases. Gastroenterology. 1992; 103:264–274. PMID: 1612333.
Article38. Streetz KL, Tacke F, Leifeld L, Wüstefeld T, Graw A, Klein C, Kamino K, Spengler U, Kreipe H, Kubicka S, Müller W, Manns MP, Trautwein C. Interleukin 6/gp130-dependent pathways are protective during chronic liver diseases. Hepatology. 2003; 38:218–229. DOI: 10.1053/jhep.2003.50268. PMID: 12830005.
Article39. Ren X, Hu B, Colletti L. Stem cell factor and its receptor, c-kit, are important for hepatocyte proliferation in wild-type and tumor necrosis factor receptor-1 knockout mice after 70% hepatectomy. Surgery. 2008; 143:790–802. DOI: 10.1016/j.surg.2008.03.021. PMID: 18549896. PMCID: 2495772.
Article40. D’Anello L, Sansone P, Storci G, Mitrugno V, D’Uva G, Chieco P, Bonafé M. Epigenetic control of the basal-like gene expression profile via Interleukin-6 in breast cancer cells. Mol Cancer. 2010; 9:300. DOI: 10.1186/1476-4598-9-300. PMCID: 3002335.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Upregulation of C-Reactive Protein by Placenta-Derived Mesenchymal Stem Cells Promotes Angiogenesis in A Rat Model with Cirrhotic Liver
- Advanced Research on Stem Cell Therapy for Hepatic Diseases: Potential Implications of a Placenta-derived Mesenchymal Stem Cell-based Strategy
- Inhibitory Effect of Tetrandrine on Extracellular Matrix Deposition in Rat Hepatic Fibrosis
- Role of Bone Marrow Mesenchymal Stem Cells in the Treatment of CCL4 Induced Liver Fibrosis in Albino Rats: A Histological and Immunohistochemical Study
- Extracellular Signal-regulated Kinase Activation Is Required for Serine 727 Phosphorylation of STAT3 in Schwann Cells in vitro and in vivo