Cancer Res Treat.
2017 Apr;49(2):374-386. 10.4143/crt.2016.080.
Induction of Apoptosis in Intestinal Toxicity to a Histone Deacetylase Inhibitor in a Phase I Study with Pelvic Radiotherapy
- Affiliations
-
- 1Department of Oncology, Akershus University Hospital, Lørenskog, Norway. a.h.ree@medisin.uio.no
- 2Institute of Clinical Molecular Biology, Akershus University Hospital, Lørenskog, Norway.
- 3Institute of Clinical Medicine, University of Oslo, Oslo, Norway.
- 4Department of Oncology, Oslo University Hospital, Oslo, Norway.
- 5Department of Tumour Biology, Oslo University Hospital, Oslo, Norway.
- 6Department of Pathology, Akershus University Hospital, Lørenskog, Norway.
- 7Department of Gastroenterological Surgery, Oslo University Hospital, Oslo, Norway.
- KMID: 2378109
- DOI: http://doi.org/10.4143/crt.2016.080
Abstract
- PURPOSE
When integrating molecularly targeted compounds in radiotherapy, synergistic effects of the systemic agent and radiation may extend the limits of patient tolerance, increasing the demand for understanding the pathophysiological mechanisms of treatment toxicity. In this Pelvic Radiation and Vorinostat (PRAVO) study, we investigated mechanisms of adverse effects in response to the histone deacetylase (HDAC) inhibitor vorinostat (suberoylanilide hydroxamic acid, SAHA) when administered as a potential radiosensitiser.
MATERIALS AND METHODS
This phase I study for advanced gastrointestinal carcinoma was conducted in sequential patient cohorts exposed to escalating doses of vorinostat combined with standard-fractionated palliative radiotherapy to pelvic target volumes. Gene expression microarray analysis of the study patient peripheral blood mononuclear cells (PBMC) was followed by functional validation in cultured cell lines and mice treated with SAHA.
RESULTS
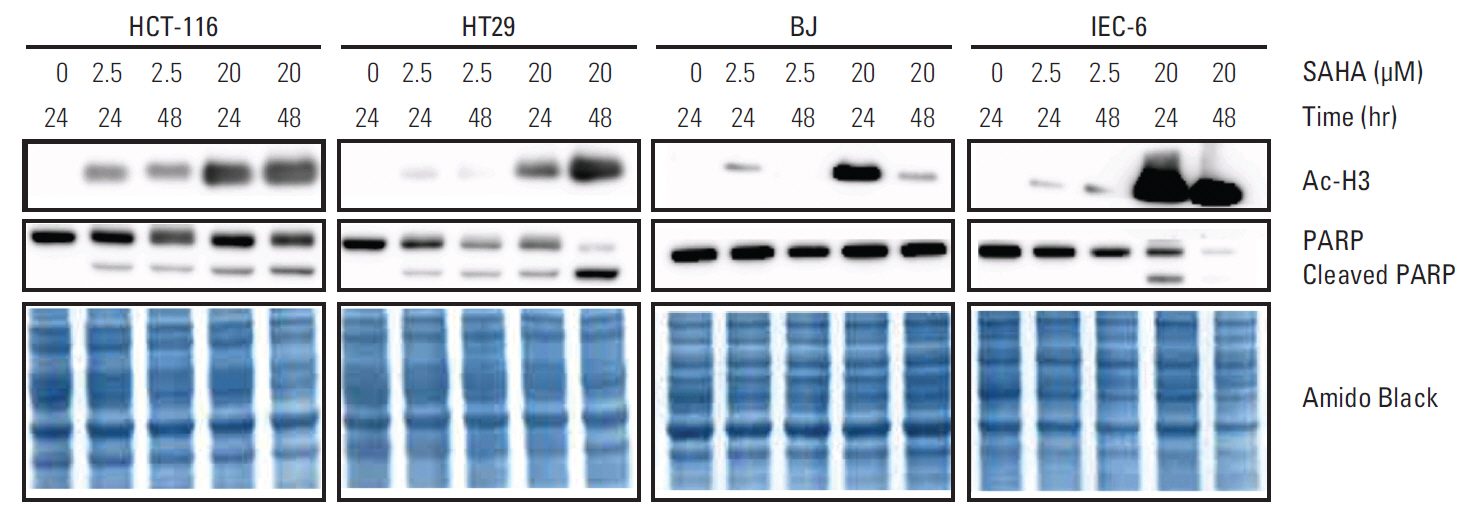
PBMC transcriptional responses to vorinostat, including induction of apoptosis, were confined to the patient cohort reporting dose-limiting intestinal toxicities. At relevant SAHA concentrations, apoptotic features (annexin V staining and caspase 3/7 activation, but not poly-(ADP-ribose)-polymerase cleavage) were observed in cultured intestinal epithelial cells. Moreover, SAHA-treated mice displayed significant weight loss.
CONCLUSION
The PRAVO study design implemented a strategy to explore treatment toxicity caused by an HDAC inhibitor when combined with radiotherapy and enabled the identification of apoptosis as a potential mechanism responsible for the dose-limiting effects of vorinostat. To the best of our knowledge, this is the first report deciphering mechanisms of normal tissue adverse effects in response to an HDAC inhibitor within a combined-modality treatment regimen.
Keyword
MeSH Terms
-
Animals
Apoptosis*
Cells, Cultured
Clinical Trials, Phase I as Topic
Cohort Studies
Drug-Related Side Effects and Adverse Reactions
Epithelial Cells
Gene Expression
Histone Deacetylase Inhibitors*
Histone Deacetylases*
Histones*
Humans
Hydroxamic Acids
Mice
Microarray Analysis
Radiotherapy*
Weight Loss
Histone Deacetylase Inhibitors
Histone Deacetylases
Histones
Hydroxamic Acids
Figure
Reference
-
References
1. Ree AH, Hollywood D. Design and conduct of early-phase radiotherapy trials with targeted therapeutics: lessons from the PRAVO experience. Radiother Oncol. 2013; 108:3–16.
Article2. Marks PA, Richon VM, Breslow R, Rifkind RA. Histone deacetylase inhibitors as new cancer drugs. Curr Opin Oncol. 2001; 13:477–83.
Article3. Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006; 5:769–84.
Article4. Stimson L, Wood V, Khan O, Fotheringham S, La Thangue NB. HDAC inhibitor-based therapies and haematological malignancy. Ann Oncol. 2009; 20:1293–302.
Article5. Khan O, La Thangue NB. HDAC inhibitors in cancer biology: emerging mechanisms and clinical applications. Immunol Cell Biol. 2012; 90:85–94.
Article6. Groselj B, Sharma NL, Hamdy FC, Kerr M, Kiltie AE. Histone deacetylase inhibitors as radiosensitisers: effects on DNA damage signalling and repair. Br J Cancer. 2013; 108:748–54.
Article7. Yang XJ, Seto E. HATs and HDACs: from structure, function and regulation to novel strategies for therapy and prevention. Oncogene. 2007; 26:5310–8.
Article8. Insinga A, Monestiroli S, Ronzoni S, Gelmetti V, Marchesi F, Viale A, et al. Inhibitors of histone deacetylases induce tumorselective apoptosis through activation of the death receptor pathway. Nat Med. 2005; 11:71–6.
Article9. Bolden JE, Shi W, Jankowski K, Kan CY, Cluse L, Martin BP, et al. HDAC inhibitors induce tumor-cell-selective pro-apoptotic transcriptional responses. Cell Death Dis. 2013; 4:e519.
Article10. West AC, Johnstone RW. New and emerging HDAC inhibitors for cancer treatment. J Clin Invest. 2014; 124:30–9.
Article11. Ree AH, Meltzer S, Flatmark K, Dueland S, Kalanxhi E. Biomarkers of treatment toxicity in combined-modality cancer therapies with radiation and systemic drugs: study design, multiplex methods, molecular networks. Int J Mol Sci. 2014; 15:22835–56.12. Ree AH, Dueland S, Folkvord S, Hole KH, Seierstad T, Johansen M, et al. Vorinostat, a histone deacetylase inhibitor, combined with pelvic palliative radiotherapy for gastrointestinal carcinoma: the Pelvic Radiation and Vorinostat (PRAVO) phase 1 study. Lancet Oncol. 2010; 11:459–64.
Article13. Flatmark K, Nome RV, Folkvord S, Bratland A, Rasmussen H, Ellefsen MS, et al. Radiosensitization of colorectal carcinoma cell lines by histone deacetylase inhibition. Radiat Oncol. 2006; 1:25.
Article14. Ree AH, Folkvord S, Flatmark K. HDAC2 deficiency and histone acetylation. Nat Genet. 2008; 40:812–3.
Article15. Folkvord S, Ree AH, Furre T, Halvorsen T, Flatmark K. Radiosensitization by SAHA in experimental colorectal carcinoma models-in vivo effects and relevance of histone acetylation status. Int J Radiat Oncol Biol Phys. 2009; 74:546–52.
Article16. Saelen MG, Ree AH, Kristian A, Fleten KG, Furre T, Hektoen HH, et al. Radiosensitization by the histone deacetylase inhibitor vorinostat under hypoxia and with capecitabine in experimental colorectal carcinoma. Radiat Oncol. 2012; 7:165.
Article17. Lane AA, Chabner BA. Histone deacetylase inhibitors in cancer therapy. J Clin Oncol. 2009; 27:5459–68.
Article18. Bratland A, Dueland S, Hollywood D, Flatmark K, Ree AH. Gastrointestinal toxicity of vorinostat: reanalysis of phase 1 study results with emphasis on dose-volume effects of pelvic radiotherapy. Radiat Oncol. 2011; 6:33.
Article19. Begg AC, Stewart FA, Vens C. Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer. 2011; 11:239–53.
Article20. Ree AH, Saelen MG, Kalanxhi E, Ostensen IH, Schee K, Roe K, et al. Biomarkers of histone deacetylase inhibitor activity in a phase 1 combined-modality study with radiotherapy. PLoS One. 2014; 9:e89750.
Article21. Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001; 98:5116–21.
Article22. Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009; 4:44–57.
Article23. Tahiri A, Roe K, Ree AH, de Wijn R, Risberg K, Busch C, et al. Differential inhibition of ex-vivo tumor kinase activity by vemurafenib in BRAF(V600E) and BRAF wild-type metastatic malignant melanoma. PLoS One. 2013; 8:e72692.
Article24. Moolenbeek C, Ruitenberg EJ. The "Swiss roll": a simple technique for histological studies of the rodent intestine. Lab Anim. 1981; 15:57–9.
Article25. Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013; 31:51–72.
Article26. Kelly WK, O'Connor OA, Krug LM, Chiao JH, Heaney M, Curley T, et al. Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol. 2005; 23:3923–31.
Article27. Xargay-Torrent S, Lopez-Guerra M, Saborit-Villarroya I, Rosich L, Campo E, Roue G, et al. Vorinostat-induced apoptosis in mantle cell lymphoma is mediated by acetylation of proapoptotic BH3-only gene promoters. Clin Cancer Res. 2011; 17:3956–68.
Article28. Grossmann J, Walther K, Artinger M, Rummele P, Woenckhaus M, Scholmerich J. Induction of apoptosis before shedding of human intestinal epithelial cells. Am J Gastroenterol. 2002; 97:1421–8.
Article29. Roostaee A, Guezguez A, Beausejour M, Simoneau A, Vachon PH, Levy E, et al. Histone deacetylase inhibition impairs normal intestinal cell proliferation and promotes specific gene expression. J Cell Biochem. 2015; 116:2695–708.
Article30. Kavanagh BD, Pan CC, Dawson LA, Das SK, Li XA, Ten Haken RK, et al. Radiation dose-volume effects in the stomach and small bowel. Int J Radiat Oncol Biol Phys. 2010; 76(3 Suppl):S101–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Apoptotic Effect of Combination Treatment with a Proteasome Inhibitor, Lactacystin, and a Histone Deacetylase Inhibitor, Trichostatin A, on MCF-7 Cells
- Anti-Cancer Effect of 3-(4-dimethylamino phenyl)-N-hydroxy-2-propenamide in MCF-7 Human Breast Cancer
- Improved Therapeutic Effect against Leukemia by a Combination of the Histone Methyltransferase Inhibitor Chaetocin and the Histone Deacetylase Inhibitor Trichostatin A
- Combination Therapy of Bortezomib (PS-341) and SAHA (Vorinostat) in Non-Small Cell Lung Cancer Cell Lines
- The effect of combined treatment with cisplatin and histone deacetylase inhibitors on HeLa cells