J Gynecol Oncol.
2010 Dec;21(4):262-268. 10.3802/jgo.2010.21.4.262.
The effect of combined treatment with cisplatin and histone deacetylase inhibitors on HeLa cells
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. jhnam@amc.seoul.kr
- 2Intergrative Omics Research Center, Korea Research Institute for Biology and Biotechnology, Daejeon, Korea.
- 3Department of Western Medicine, Life University School of Oriental Medicine, Gardena, CA , USA.
- KMID: 2173551
- DOI: http://doi.org/10.3802/jgo.2010.21.4.262
Abstract
OBJECTIVE
To investigate the combined effects of cisplatin and the histone deacetylase (HDAC) inhibitors suberoylanilide hydroxamic acid (SAHA) or sirtinol on HeLa cells and assess the mechanism underlying HDAC inhibitor-cisplatin synergy.
METHODS
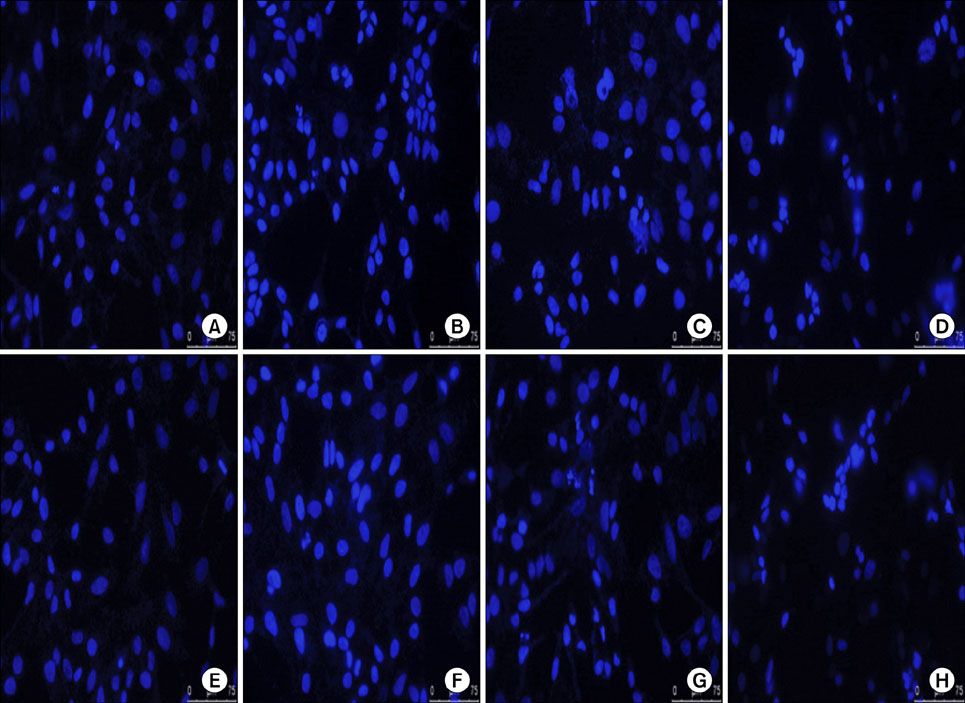
The antineoplastic actions of cisplatin, SAHA and sirtinol, alone and in combination, were evaluated using the tetrazolium dye-based MTT cell proliferation assay, DAPI nuclear staining and cytotoxicity analysis.
RESULTS
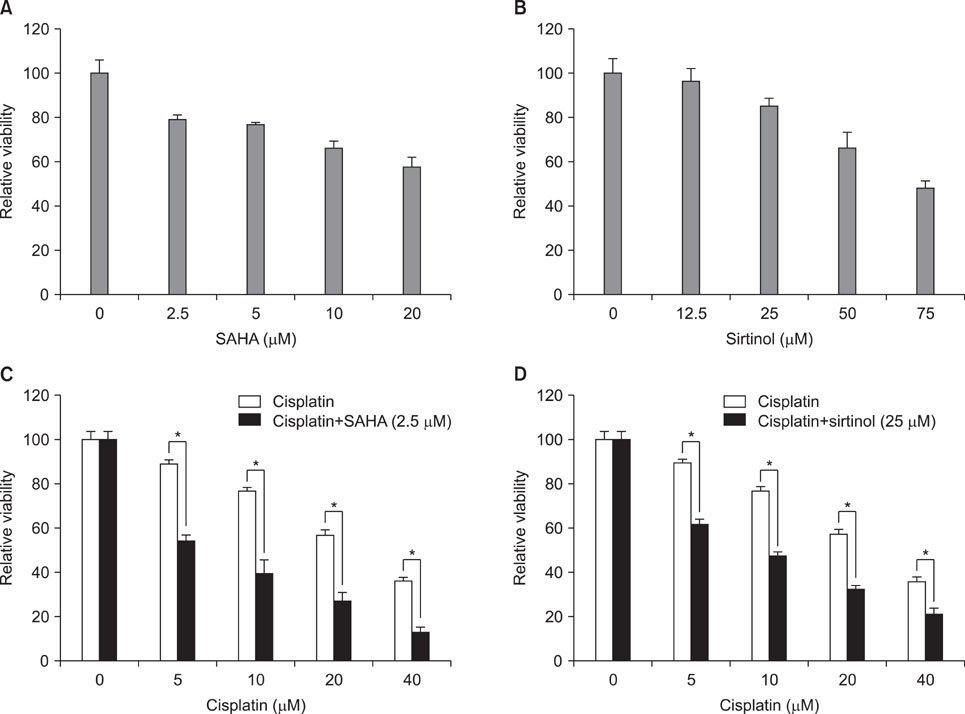
Exposure to cisplatin, SAHA or sirtinol alone induced a dose-dependent reduction in HeLa cell viability. Combined treatment with cisplatin and SAHA or sirtinol was significantly more cytotoxic than cisplatin alone. Individually, cisplatin, SAHA and sirtinol activated caspase-3 and induced apoptosis, but the effects of combined treatment were greater. Importantly, both HDAC inhibitors dose-dependently inhibited the expression of the antiapoptotic proteins Bcl-2 and x-linked inhibitor of apoptosis protein (XIAP).
CONCLUSION
The combination of cisplatin and SAHA or sirtinol had synergistic effect on the HeLa cell viability. This potentiation of cisplatin activity was associated with HDAC inhibitor-mediated down-regulation of Bcl-2 and XIAP. These may result from the relaxation of chromatin by these HDAC inhibitors that increase cisplatin sensitivity by enhancing the accessibility of DNA to cisplatin and transcriptional regulators.
MeSH Terms
-
Apoptosis
Benzamides
Caspase 3
Cell Proliferation
Chromatin
Cisplatin
DNA
Down-Regulation
HeLa Cells
Histone Deacetylase Inhibitors
Histone Deacetylases
Histones
Humans
Hydroxamic Acids
Indoles
Naphthols
Proteins
Relaxation
Uterine Cervical Neoplasms
X-Linked Inhibitor of Apoptosis Protein
Benzamides
Caspase 3
Chromatin
Cisplatin
DNA
Histone Deacetylase Inhibitors
Histone Deacetylases
Histones
Hydroxamic Acids
Indoles
Naphthols
Proteins
X-Linked Inhibitor of Apoptosis Protein
Figure
Reference
-
1. Khochbin S, Verdel A, Lemercier C, Seigneurin-Berny D. Functional significance of histone deacetylase diversity. Curr Opin Genet Dev. 2001. 11:162–166.2. Marmorstein R, Roth SY. Histone acetyltransferases: function, structure, and catalysis. Curr Opin Genet Dev. 2001. 11:155–161.3. Roth SY, Denu JM, Allis CD. Histone acetyltransferases. Annu Rev Biochem. 2001. 70:81–120.4. Lee DY, Hayes JJ, Pruss D, Wolffe AP. A positive role for histone acetylation in transcription factor access to nucleosomal DNA. Cell. 1993. 72:73–84.5. Grunstein M. Histone acetylation in chromatin structure and transcription. Nature. 1997. 389:349–352.6. Struhl K. Histone acetylation and transcriptional regulatory mechanisms. Genes Dev. 1998. 12:599–606.7. Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000. 403:41–45.8. Marks PA, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer. 2001. 1:194–202.9. Zhang XD, Gillespie SK, Borrow JM, Hersey P. The histone deacetylase inhibitor suberic bishydroxamate regulates the expression of multiple apoptotic mediators and induces mitochondria-dependent apoptosis of melanoma cells. Mol Cancer Ther. 2004. 3:425–435.10. Rosato RR, Maggio SC, Almenara JA, Payne SG, Atadja P, Spiegel S, et al. The histone deacetylase inhibitor LAQ824 induces human leukemia cell death through a process involving XIAP down-regulation, oxidative injury, and the acid sphingomyelinase-dependent generation of ceramide. Mol Pharmacol. 2006. 69:216–225.11. Mitsiades CS, Mitsiades NS, McMullan CJ, Poulaki V, Shringarpure R, Hideshima T, et al. Transcriptional signature of histone deacetylase inhibition in multiple myeloma: biological and clinical implications. Proc Natl Acad Sci USA. 2004. 101:540–545.12. Peart MJ, Smyth GK, van Laar RK, Bowtell DD, Richon VM, Marks PA, et al. Identification and functional significance of genes regulated by structurally different histone deacetylase inhibitors. Proc Natl Acad Sci U S A. 2005. 102:3697–3702.13. Moore PS, Barbi S, Donadelli M, Costanzo C, Bassi C, Palmieri M, et al. Gene expression profiling after treatment with the histone deacetylase inhibitor trichostatin A reveals altered expression of both pro- and anti-apoptotic genes in pancreatic adenocarcinoma cells. Biochim Biophys Acta. 2004. 1693:167–176.14. Duan H, Heckman CA, Boxer LM. Histone deacetylase inhibitors down-regulate bcl-2 expression and induce apoptosis in t(14;18) lymphomas. Mol Cell Biol. 2005. 25:1608–1619.15. Ruefli AA, Ausserlechner MJ, Bernhard D, Sutton VR, Tainton KM, Kofler R, et al. The histone deacetylase inhibitor and chemotherapeutic agent suberoylanilide hydroxamic acid (SAHA) induces a cell-death pathway characterized by cleavage of Bid and production of reactive oxygen species. Proc Natl Acad Sci U S A. 2001. 98:10833–10838.16. Rosato RR, Almenara JA, Dai Y, Grant S. Simultaneous activation of the intrinsic and extrinsic pathways by histone deacetylase (HDAC) inhibitors and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) synergistically induces mitochondrial damage and apoptosis in human leukemia cells. Mol Cancer Ther. 2003. 2:1273–1284.17. Marks PA, Richon VM, Rifkind RA. Histone deacetylase inhibitors: inducers of differentiation or apoptosis of transformed cells. J Natl Cancer Inst. 2000. 92:1210–1216.18. Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006. 5:769–784.19. Butler LM, Agus DB, Scher HI, Higgins B, Rose A, Cordon-Cardo C, et al. Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, suppresses the growth of prostate cancer cells in vitro and in vivo. Cancer Res. 2000. 60:5165–5170.20. Sandor V, Bakke S, Robey RW, Kang MH, Blagosklonny MV, Bender J, et al. Phase I trial of the histone deacetylase inhibitor, depsipeptide (FR901228, NSC 630176), in patients with refractory neoplasms. Clin Cancer Res. 2002. 8:718–728.21. Duvic M, Zhang C. Clinical and laboratory experience of vorinostat (suberoylanilide hydroxamic acid) in the treatment of cutaneous T-cell lymphoma. Br J Cancer. 2006. 95:S1. S13–S19.22. O'Connor OA. Clinical experience with the novel histone deacetylase inhibitor vorinostat (suberoylanilide hydroxamic acid) in patients with relapsed lymphoma. Br J Cancer. 2006. 95:S1. S7–S12.23. Ruefli AA, Bernhard D, Tainton KM, Kofler R, Smyth MJ, Johnstone RW. Suberoylanilide hydroxamic acid (SAHA) overcomes multidrug resistance and induces cell death in P-glycoprotein-expressing cells. Int J Cancer. 2002. 99:292–298.24. Chu F, Chou PM, Zheng X, Mirkin BL, Rebbaa A. Control of multidrug resistance gene mdr1 and cancer resistance to chemotherapy by the longevity gene sirt1. Cancer Res. 2005. 65:10183–10187.25. Kim MS, Blake M, Baek JH, Kohlhagen G, Pommier Y, Carrier F. Inhibition of histone deacetylase increases cytotoxicity to anticancer drugs targeting DNA. Cancer Res. 2003. 63:7291–7300.26. Deveraux QL, Roy N, Stennicke HR, Van Arsdale T, Zhou Q, Srinivasula SM, et al. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J. 1998. 17:2215–2223.27. Deveraux QL, Leo E, Stennicke HR, Welsh K, Salvesen GS, Reed JC. Cleavage of human inhibitor of apoptosis protein XIAP results in fragments with distinct specificities for caspases. EMBO J. 1999. 18:5242–5251.28. Asselin E, Mills GB, Tsang BK. XIAP regulates Akt activity and caspase-3-dependent cleavage during cisplatin-induced apoptosis in human ovarian epithelial cancer cells. Cancer Res. 2001. 61:1862–1868.29. Gagnon V, Van Themsche C, Turner S, Leblanc V, Asselin E. Akt and XIAP regulate the sensitivity of human uterine cancer cells to cisplatin, doxorubicin and taxol. Apoptosis. 2008. 13:259–271.30. Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984. 22:27–55.31. Papeleu P, Vanhaecke T, Elaut G, Vinken M, Henkens T, Snykers S, et al. Differential effects of histone deacetylase inhibitors in tumor and normal cells-what is the toxicological relevance? Crit Rev Toxicol. 2005. 35:363–378.32. Reed E, Yuspa SH, Zwelling LA, Ozols RF, Poirier MC. Quantitation of cis-diamminedichloroplatinum II (cisplatin)-DNA-intrastrand adducts in testicular and ovarian cancer patients receiving cisplatin chemotherapy. J Clin Invest. 1986. 77:545–550.33. Anderson ME. Glutathione: an overview of biosynthesis and modulation. Chem Biol Interact. 1998. 111-112:1–14.34. Schroder CP, Godwin AK, O'Dwyer PJ, Tew KD, Hamilton TC, Ozols RF. Glutathione and drug resistance. Cancer Invest. 1996. 14:158–168.35. Ishikawa T, Ali-Osman F. Glutathione-associated cis-diamminedichloroplatinum( II) metabolism and ATP-dependent efflux from leukemia cells: molecular characterization of glutathione-platinum complex and its biological significance. J Biol Chem. 1993. 268:20116–20125.36. Meister A. Glutathione metabolism and its selective modification. J Biol Chem. 1988. 263:17205–17208.37. Mese H, Sasaki A, Alcalde RE, Nakayama S, Matsumura T. Regulation of apoptosis reduction in the cisplatin-resistant A431 cell line by Bcl-2 and CPP32. Chemotherapy. 2000. 46:69–76.38. Miyake H, Hara I, Yamanaka K, Arakawa S, Kamidono S. Synergistic enhancement of resistance to cisplatin in human bladder can cer cells by overexpression of mutant-type p53 and Bcl-2. J Urol. 1999. 162:2176–2181.39. Miyake H, Hanada N, Nakamura H, Kagawa S, Fujiwara T, Hara I, et al. Overexpression of Bcl-2 in bladder cancer cells inhibits apoptosis induced by cisplatin and adenoviral-mediated p53 gene transfer. Oncogene. 1998. 16:933–943.40. Zangemeister-Wittke U, Schenker T, Luedke GH, Stahel RA. Synergistic cytotoxicity of bcl-2 antisense oligodeoxynucleotides and etoposide, doxorubicin and cisplatin on small-cell lung cancer cell lines. Br J Cancer. 1998. 78:1035–1042.41. Simonian PL, Grillot DA, Merino R, Nunez G. Bax can antagonize Bcl-XL during etoposide and cisplatin-induced cell death independently of its heterodimerization with Bcl-XL. J Biol Chem. 1996. 271:22764–22772.42. Hockenbery DM, Oltvai ZN, Yin XM, Milliman CL, Korsmeyer SJ. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993. 75:241–251.43. Voehringer DW. BCL-2 and glutathione: alterations in cellular redox state that regulate apoptosis sensitivity. Free Radic Biol Med. 1999. 27:945–950.44. Yao MK, Desilets H, Charles MT, Boulanger R, Tweddell RJ. Effect of mycorrhization on the accumulation of rishitin and solavetivone in potato plantlets challenged with Rhizoctonia solani. Mycorrhiza. 2003. 13:333–336.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Activation of ATM-dependent DNA Damage Signal Pathway by a Histone Deacetylase Inhibitor, Trichostatin A
- Leptomycin B Increases Radiosensitization by Trichostain A in HeLa Cells
- IL-4 and HDAC Inhibitors Suppress Cyclooxygenase-2 Expression in Human Follicular Dendritic Cells
- Apoptotic Effect of Combination Treatment with a Proteasome Inhibitor, Lactacystin, and a Histone Deacetylase Inhibitor, Trichostatin A, on MCF-7 Cells
- The Role of Histone Acetylation in Mesenchymal Stem Cell Differentiation