Korean J Physiol Pharmacol.
2017 Mar;21(2):267-273. 10.4196/kjpp.2017.21.2.267.
The oncogenic effects of p53-inducible gene 3 (PIG3) in colon cancer cells
- Affiliations
-
- 1Laboratory of Genomic Instability and Cancer Therapeutics, Cancer Mutation Research Center, Chosun University School of Medicine, Gwangju 61452, Korea. jhlee75@chosun.ac.kr
- 2Department of Premedical Sciences, Chosun University School of Medicine, Gwangju 61452, Korea.
- 3Department of Internal Medicine, Hemato-oncology, Chosun University School of Medicine, Gwangju 61452, Korea. sgpark@chosun.ac.kr
- 4Department of Neurosurgery, Chosun University School of Medicine, Gwangju 61452, Korea. chosunns@chosun.ac.kr
- 5Department of Cellular and Molecular Medicine, Chosun University School of Medicine, Gwangju 61452, Korea.
- KMID: 2371046
- DOI: http://doi.org/10.4196/kjpp.2017.21.2.267
Abstract
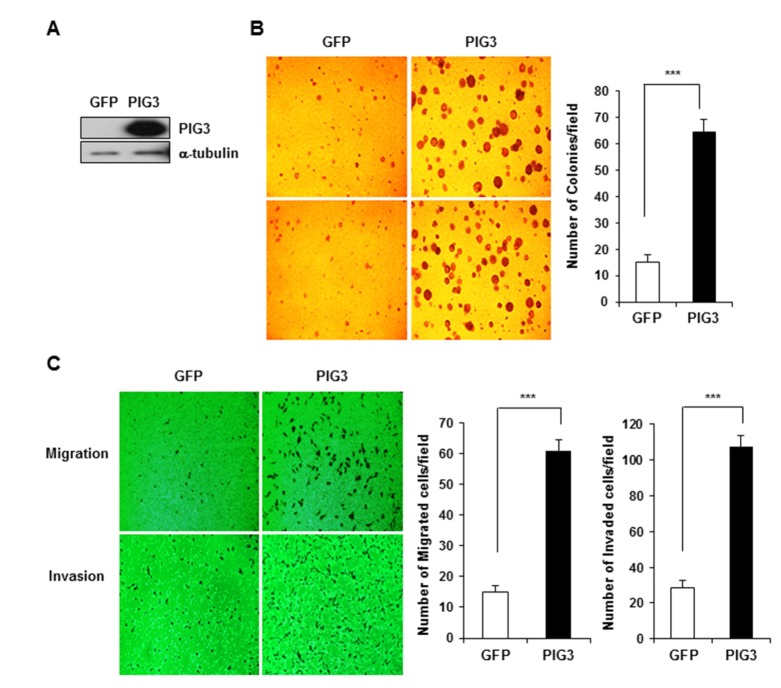
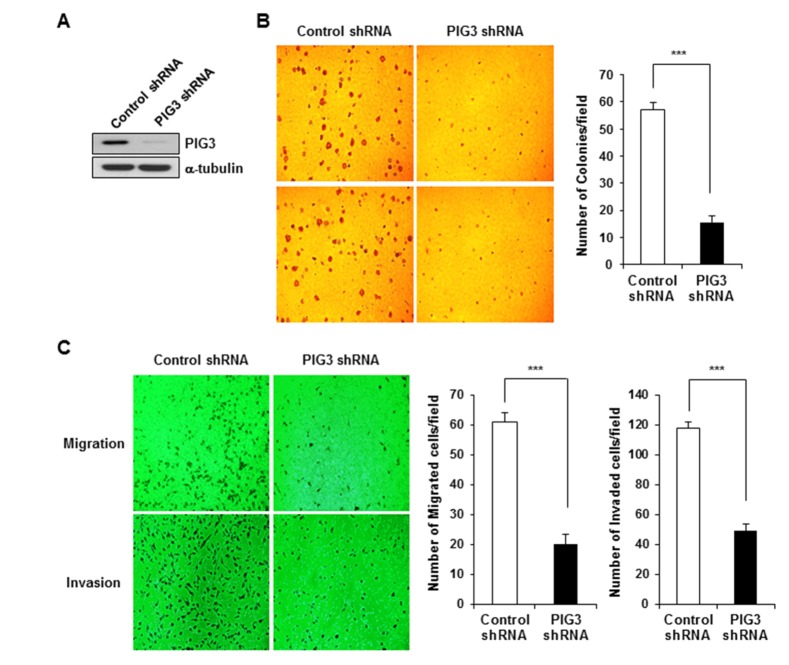
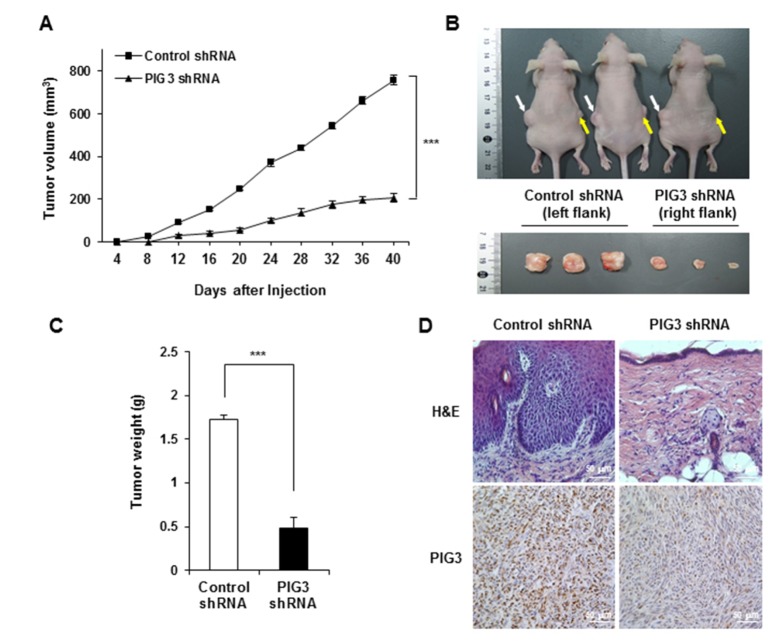
- The p53-inducible gene 3 (PIG3), initially identified as a gene downstream of p53, plays an important role in the apoptotic process triggered by p53-mediated reactive oxygen species (ROS) production. Recently, several studies have suggested that PIG3 may play a role in various types of cancer. However, the functional significance of PIG3 in cancer remains unclear. Here, we found that PIG3 was highly expressed in human colon cancer cell lines compared to normal colonderived fibroblasts. Therefore, we attempted to elucidate the functional role of PIG3 in colon cancer. PIG3 overexpression increases the colony formation, migration and invasion ability of HCT116 colon cancer cells. Conversely, these tumorigenic abilities were significantly decreased in in vitro studies with PIG3 knockdown HCT116 cells. PIG3 knockdown also attenuated the growth of mouse xenograft tumors. These results demonstrate that PIG3 is associated with the tumorigenic potential of cancer cells, both in vitro and in vivo, and could play a key oncogenic role in colon cancer.
Keyword
MeSH Terms
Figure
Reference
-
1. Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B. A model for p53-induced apoptosis. Nature. 1997; 389:300–305. PMID: 9305847.
Article2. Johnson TM, Yu ZX, Ferrans VJ, Lowenstein RA, Finkel T. Reactive oxygen species are downstream mediators of p53-dependent apoptosis. Proc Natl Acad Sci U S A. 1996; 93:11848–11852. PMID: 8876226.
Article3. Contente A, Dittmer A, Koch MC, Roth J, Dobbelstein M. A polymorphic microsatellite that mediates induction of PIG3 by p53. Nat Genet. 2002; 30:315–320. PMID: 11919562.
Article4. Porté S, Valencia E, Yakovtseva EA, Borràs E, Shafqat N, Debreczeny JE, Pike AC, Oppermann U, Farrés J, Fita I, Parés X. Three-dimensional structure and enzymatic function of proapoptotic human p53-inducible quinone oxidoreductase PIG3. J Biol Chem. 2009; 284:17194–17205. PMID: 19349281.
Article5. Siegel D, Gustafson DL, Dehn DL, Han JY, Boonchoong P, Berliner LJ, Ross D. NAD(P)H:quinone oxidoreductase 1: role as a superoxide scavenger. Mol Pharmacol. 2004; 65:1238–1247. PMID: 15102952.
Article6. Kang MY, Kim HB, Piao C, Lee KH, Hyun JW, Chang IY, You HJ. The critical role of catalase in prooxidant and antioxidant function of p53. Cell Death Differ. 2013; 20:117–129. PMID: 22918438.
Article7. Long XH, Zhao ZQ, He XP, Wang HP, Xu QZ, An J, Bai B, Sui JL, Zhou PK. Dose-dependent expression changes of early response genes to ionizing radiation in human lymphoblastoid cells. Int J Mol Med. 2007; 19:607–615. PMID: 17334636.
Article8. Lee JH, Kang Y, Khare V, Jin ZY, Kang MY, Yoon Y, Hyun JW, Chung MH, Cho SI, Jun JY, Chang IY, You HJ. The p53-inducible gene 3 (PIG3) contributes to early cellular response to DNA damage. Oncogene. 2010; 29:1431–1450. PMID: 20023697.
Article9. Li B, Shang ZF, Yin JJ, Xu QZ, Liu XD, Wang Y, Zhang SM, Guan H, Zhou PK. PIG3 functions in DNA damage response through regulating DNA-PKcs homeostasis. Int J Biol Sci. 2013; 9:425–434. PMID: 23678292.
Article10. Ito M, Nishiyama H, Watanabe J, Kawanishi H, Takahashi T, Kamoto T, Habuchi T, Ogawa O. Association of the PIG3 promoter polymorphism with invasive bladder cancer in a Japanese population. Jpn J Clin Oncol. 2006; 36:116–120. PMID: 16418181.
Article11. Guan X, Liu Z, Wang L, Wang LE, Sturgis EM, Wei Q. Functional repeats (TGYCC)n in the p53-inducible gene 3 (PIG3) promoter and susceptibility to squamous cell carcinoma of the head and neck. Carcinogenesis. 2013; 34:812–817. PMID: 23241165.12. Xu J, Cai J, Jin X, Yang J, Shen Q, Ding X, Liang Y. PIG3 plays an oncogenic role in papillary thyroid cancer by activating the PI3K/AKT/PTEN pathway. Oncol Rep. 2015; 34:1424–1430. PMID: 26133772.
Article13. Nicholls CD, Shields MA, Lee PW, Robbins SM, Beattie TL. UV-dependent alternative splicing uncouples p53 activity and PIG3 gene function through rapid proteolytic degradation. J Biol Chem. 2004; 279:24171–24178. PMID: 15067011.
Article14. Flatt PM, Polyak K, Tang LJ, Scatena CD, Westfall MD, Rubinstein LA, Yu J, Kinzler KW, Vogelstein B, Hill DE, Pietenpol JA. p53-dependent expression of PIG3 during proliferation, genotoxic stress,and reversible growth arrest. Cancer Lett. 2000; 156:63–72. PMID: 10840161.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- PIG3 Regulates p53 Stability by Suppressing Its MDM2-Mediated Ubiquitination
- The Synergistic Cell Killing Effects by the Transduction of the w-p53 Gene and 5-FU Administration in Colon Cancer Cell Lines
- Effects of Wild - type p53 Gene Transfection into Human Colon Cancer Cell Line
- Expression of bcl-2 Protein in Colorectal Adenoma and Adenocarcinoma and its Relationship with p53 and Apoptosis
- Mutation of p53 Gene and Detection of Human Papillomavirus DNA in Larynx and Pharynx Cancers