Nat Prod Sci.
2016 Dec;22(4):299-306. 10.20307/nps.2016.22.4.299.
HPLC analysis of Phenolic Substances and Anti-Alzheimer's Activity of Korean Quercus Species
- Affiliations
-
- 1Department of Agro-industrial Technology, Lambung Mangkurat University, Banjarbaru 70714, Indonesia.
- 2Department of Forest Science, Sangji University, Wonju 26339, Korea.
- 3Department of Food Science and Nutrition, Pukyong National University, Busan 48513, Korea.
- 4Southeast Medi-Chem Institute, Busan 48287, Korea.
- 5Department of Pharmaceutical Engineering, Sangji University, Wonju 26339, Korea. hjpark@sangji.ac.kr
- KMID: 2366700
- DOI: http://doi.org/10.20307/nps.2016.22.4.299
Abstract
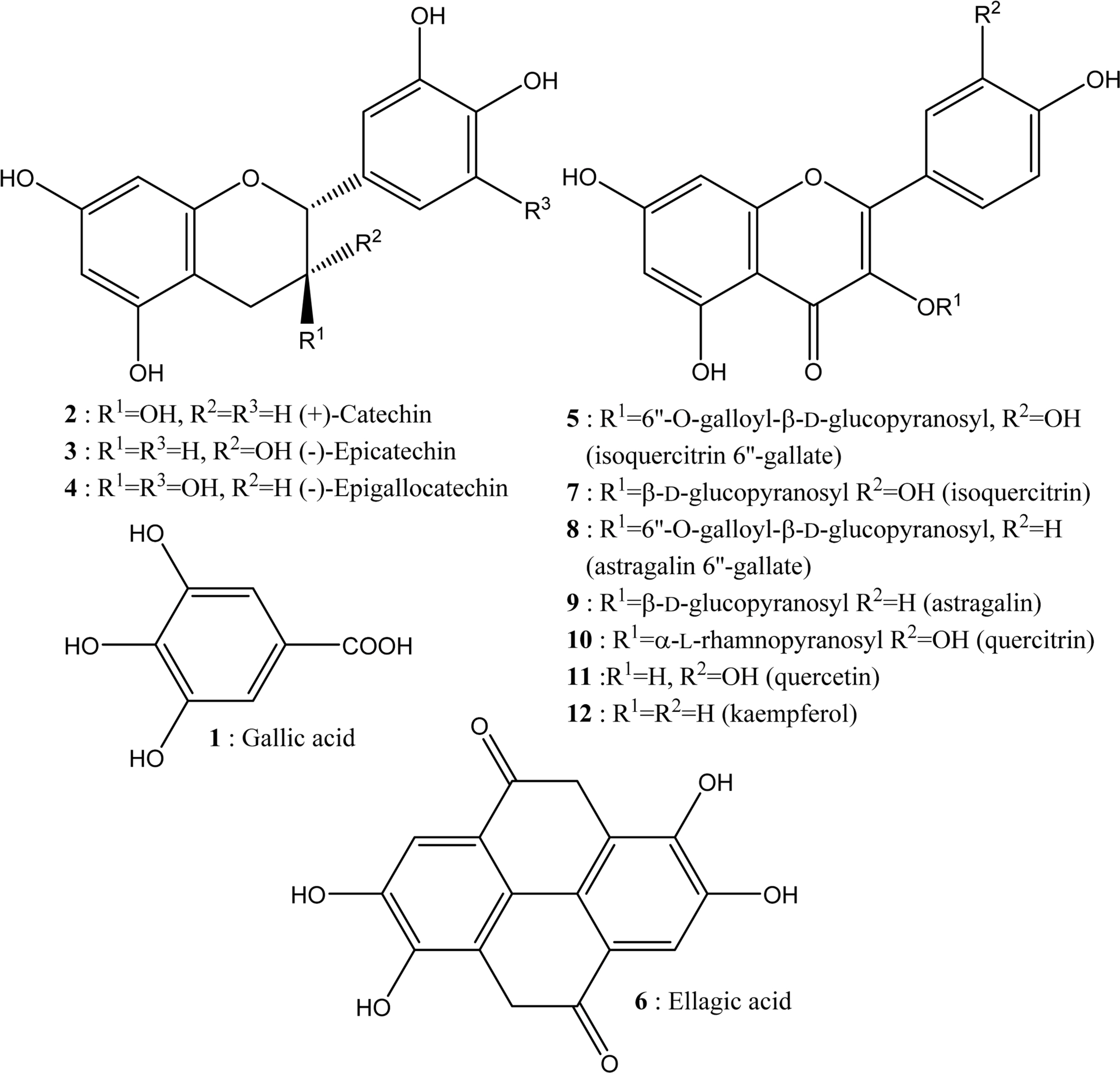
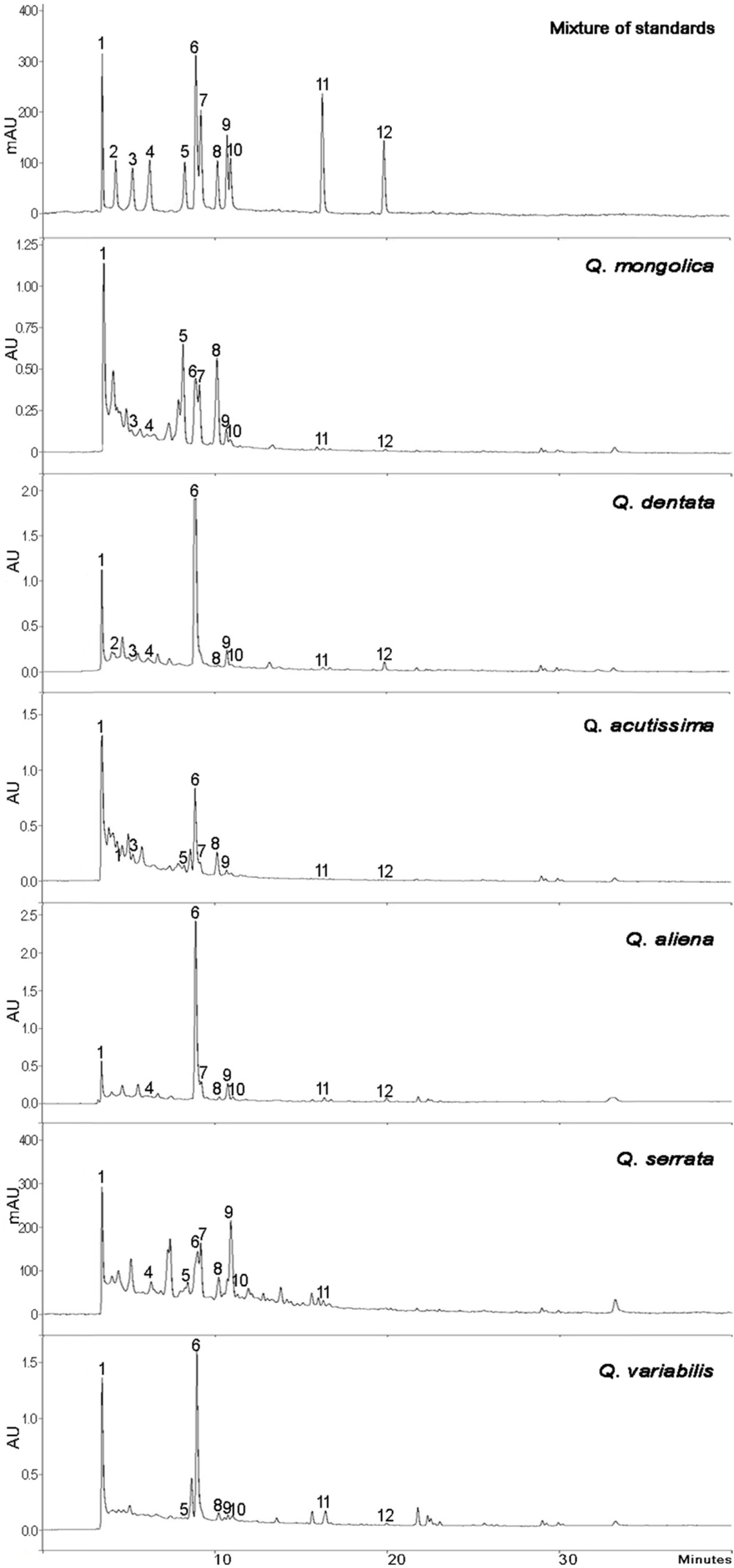
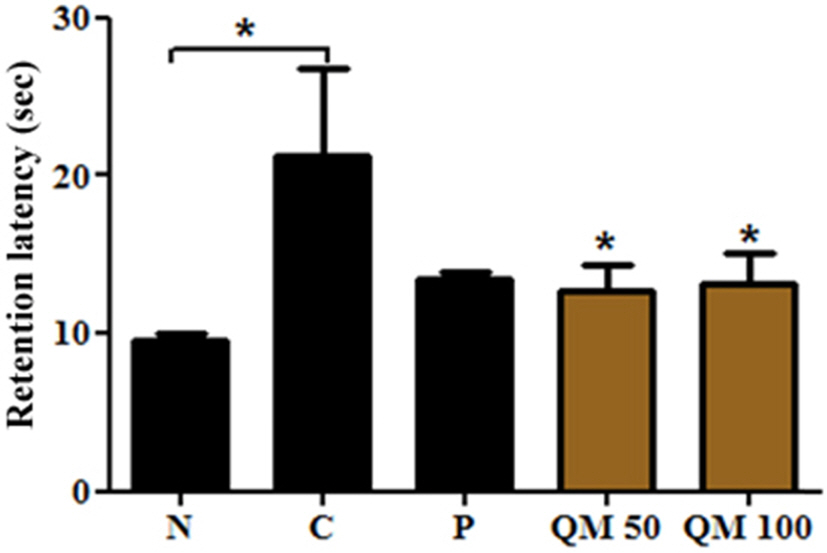
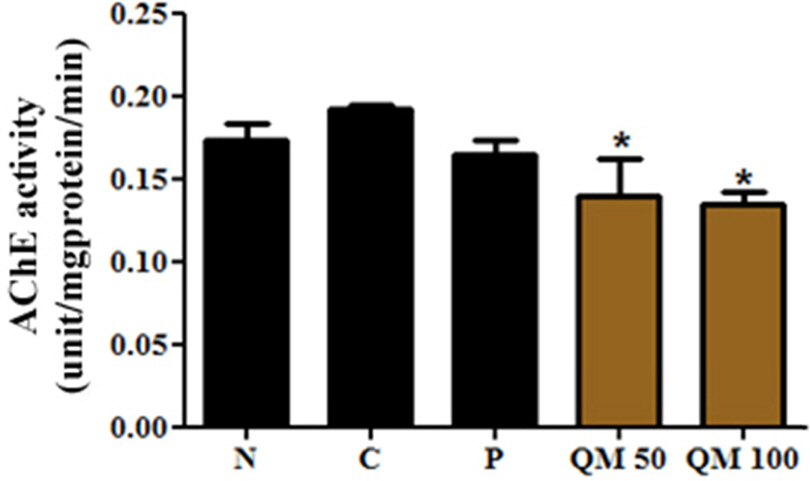
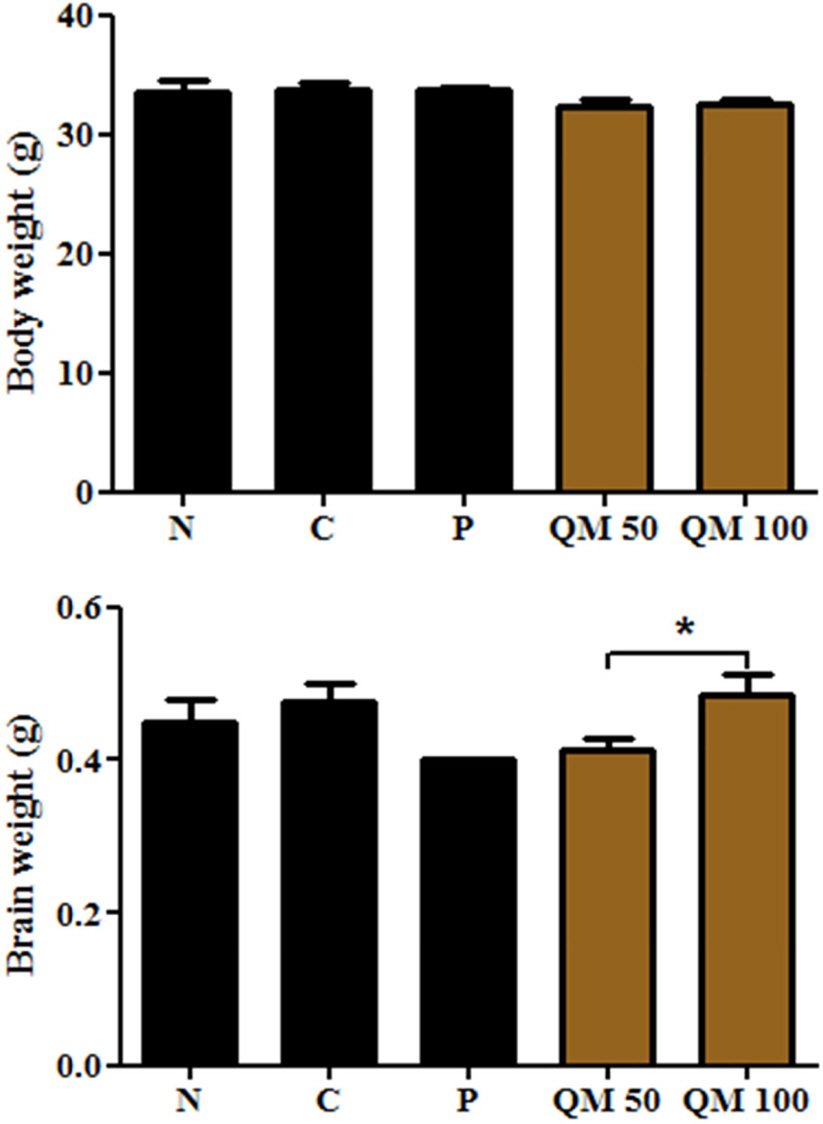
- This study aimed to establish the quantitative method to analyze the content of peroxynitrite-scavengers belonging to polyphenols in six Korean Quercus species (Quercus mongolica, Q. dentata, Q. acutissima, Q. alienta, Q. serrata, and Q. variabilis) by HPLC. The twelve peroxynitrite-scavengers, flavanols (catechins: (+)-catechin, (−)-epicatechin, and (−)-epigallocatechin), flavonols (kaempferol and quercetin), flavonol glycosides (astragalin, quercitrin, and isoquercitrin), flavonol acylated glycosides (astragalin 6"³-gallate and isoquercitrin 6"³-gallate), gallic acid and its dimer (ellagic acid) were analyzed by HPLC. Further, anti-Alzheimer's activity was assayed in a passive avoidance testusing mice by measuring the retention latency (sec), the concentration of acetylcholine (ACh), and acetylcholinesterase (AChE) activity. Simultaneous analysis of the extracts of the six Quercus leaves was achieved on a Capcell C18 column (5 µm, 250 mm × 4.6 mm i.d.) with a gradient elution of 0.05% HAc and 0.05% HAc in CH₃CN. In the extract of Q. mongolica leaves, the content of gallic acid (32.53 mg/g), (+)-catechin (28.78 mg/g), (−)-epicatehin (22.03 mg/g), astragalin 6"³-gallate (20.94 mg/g), and isoquercitrin 6"³-gallate (44.11 mg/g) and peroxynitrite-scavenging activity (ICâ‚…â‚€, 0.831 µg/ml) were high. This extract delayed the retention latency and inhibited acetylcholinesterase activity in scopolamine-induced memory impairment of mice, suggesting that it has anti-Alzheimer's activity.
MeSH Terms
Figure
Reference
-
References
(1). Kim J. G.., Bae Y. S.Chemical constituents of domestic Quercus spp. Leaves. Mokchae Konghak. 2006. 34:61–71.(2). Kuliev Z. A.., Vdovin A. D.., Abdullaev N. D.., Makhamatkulov A. D.., Matikov V. M.Chem. Nat. Prod. 1997. 33:642–652.(3). Sakar M. K.., Söhretogu D.., Özalp M.., Ekizoglu M.., Piancente S.., Pizza C.Turk. J. Chem. 2005. 29:555–559.(4). Zaveri N. T.Life Sci. 2006. 78:2073–2080.(5). Patcher P.., Obrosova I. G.., Mabley J. G.., Szabó C.Curr. Med. Chem. 2005. 12:267–275.(6). Zhang Y. J.., Xu Y. F.., Liu Y. H.., Yin J.., Li H. L.., Wang Q.., Wang J. Z.FASEB J. 2006. 20:131–142.(7). Ortiz G. G.., Benítez-King G. A.., Rosales-Corral S. A.., Pacheco-Moisés F. P.., Velázquez-Brizuela I. E.Curr. Neuropharmacol. 2008. 6:203–214.(8). Syad A. N.., Devi K. P.Bot. Targ. Ther. 2014. 4:11–26.(9). Moniruzzaman M.., Asaduzzaman M.., Hossain M. S.., Sarker J.., Rahman S. M. A.., Rashid M.., Rahman M. M.BMC Complement. Altern. Med. 2015. 15:403–412.(10). Nugroho A.., Rhim T. J.., Choi M. Y.., Choi J. S.., Kim Y. C.., Kim M. S.., Park H. J.Arch. Pharm. Res. 2014. 37:890–898.(11). Kooy N. W.., Royall J. A.., Ischiropoulos H.., Beckman J. S.Free Radic. Biol. Med. 1994. 16:149–156.(12). Van der Zee E. A.., Biemans B. A.., Gerkema M. P.., Daans S. J.Neurosci. Res. 2004. 78:508–519.(13). Ellman G. L.., Courtney K. D.., Andres V. Jr.., Feather-stone R. M.Biochem. Pharmacol. 1961. 7:88–95.(14). Hestrin S. J.Biol. Chem. 1949. 180:249–261.(15). Chung H. Y.., Yokozawa T.., Soung D. Y.., Kye I. S.., No J. K.., Baek B. S. J.Agric. Food Chem. 1998. 46:4484–4486.(16). Gimenez-Garzó C.., Urios A.., Agustí A.., González-López O.., Escudero-García D.., Escudero-Sanchis A.., Serra M. A.., Giner-Durán R.., Montoliu C.., Felipo V.Antioxid. Redox Signal. 2015. 22:871–877.(17). Smith M. A.., Taneda S.., Richey P. L.., Miyata S.., Yan S. D.., Stern D.., Sayre L. M.., Monnier V. M.., Perry G. Proc. Natl. Acad. Sci. U. S.A. 1994. 91:5710–5714.(18). Sayre L. M.., Zelasko D. A.., Harris P. L.., Perry G.., Salomon R. G.., Smith M. A. J.Neurochem. 1997. 68:2092–2097.(19). Perry G.., Cash A. D.., Smith M. A. J.Biomed. Biotechnol. 2002. 2:120–123.(20). Durairajan S. S.., Yuan Q.., Xie L.., Chan W. S.., Kum W. F.., Koo I.., Liu C.., Song Y.., Huang J. D.., Klein W. L.., Li M.Neurochem Int. 2008. 52:741–750.(21). Feng Y.., Yang S. G.., Du X. T.., Zhang X.., Sun X. X.., Zhao M.., Sun G. Y.., Liu R. T.Biochem. Biophys. Res. Commun. 2009. 390:1250–1254.(22). Zhang L.., Fiala M, Cashman J.., Sayre J.., Espinosa A.., Mahanian M.., Zaghi J.., Badmaev V.., Graves M. C.., Bernard G.., Rosenthal M. J.Alzheimers Dis. 2006. 10:1–7.(23). Hwang E. M.., Ryu Y. B.., Kim H. Y.., Kim D. G.., Hong S. G.., Lee J. H.., Curtis-Long M. J.., Jeong S. H.., Park J. Y.., Park K. H.Bioorg. Med. Chem. 2008. 16:6669–6674.(24). Descamps O.., Spilman P.., Zhang Q.., Libeu C. P.., Poksay K.., Gorostiza O.., Campagna J.., Jagodzinska B.., Bredesen D. E.., John V. J.Alzheimers Dis. 2013. 37:343–355.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- HPLC and GC-MS Analysis of Phenolic Substances in Acer tegmentosum
- Analysis of Phenolic Acid Content and Antioxidant Activity of Chestnut Honey from Different Regions of Korea
- Mycorrhization of Quercus spp. with Tuber huidongense and T. himalayense Collected in Korea
- Analysis of Flavonoid Composition of Korean Herbs in the Family of Compositae and their Utilization for Health
- Phytochemical Analysis of the Phenolic Fat-Suppressing Substances in the Leaves of Lactuca raddeana in 3T3-L1 Adipocytes