Nat Prod Sci.
2016 Mar;22(1):1-12. 10.20307/nps.2016.22.1.1.
Analysis of Flavonoid Composition of Korean Herbs in the Family of Compositae and their Utilization for Health
- Affiliations
-
- 1Department of Agro-industrial Technology, Faculty of Agriculture, Lambung Mangkurat University, Banjarbaru 70712, Indonesia.
- 2Department of Food Science and Nutrition, Pukyong National University, Busan 608-737, Korea.
- 3Department of Pharmaceutical Engineering, Sangji University, Wonju 220-702, Korea. hjpark@sangji.ac.kr
- KMID: 2312914
- DOI: http://doi.org/10.20307/nps.2016.22.1.1
Abstract
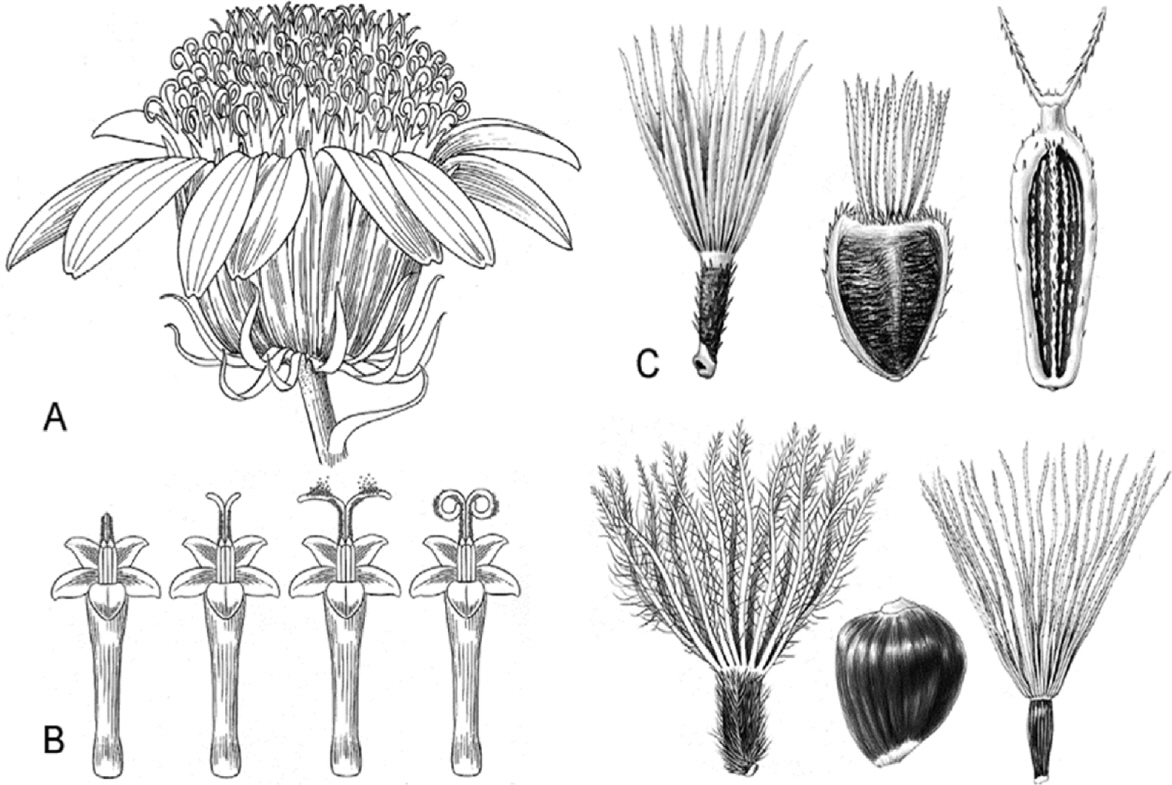
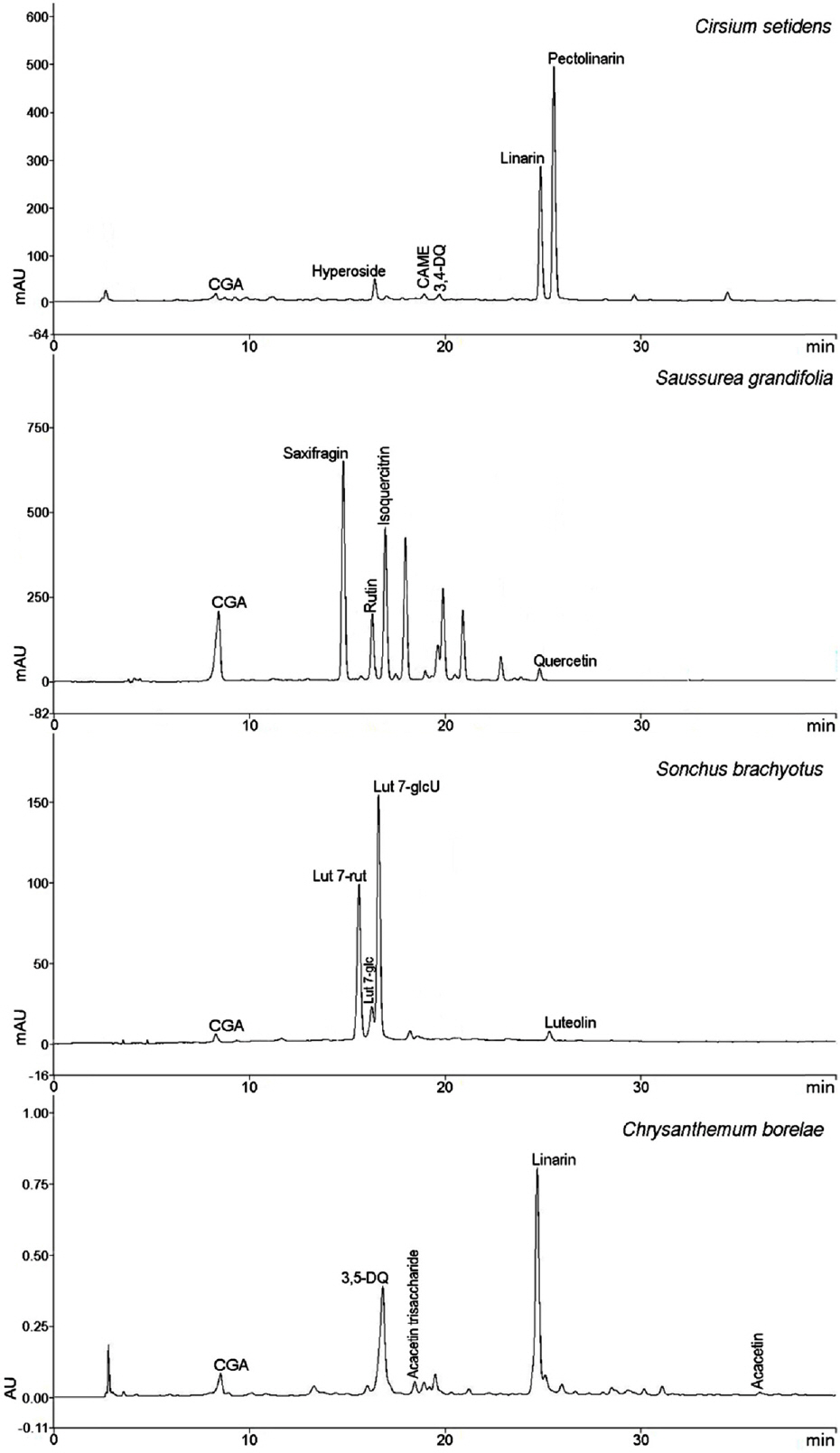
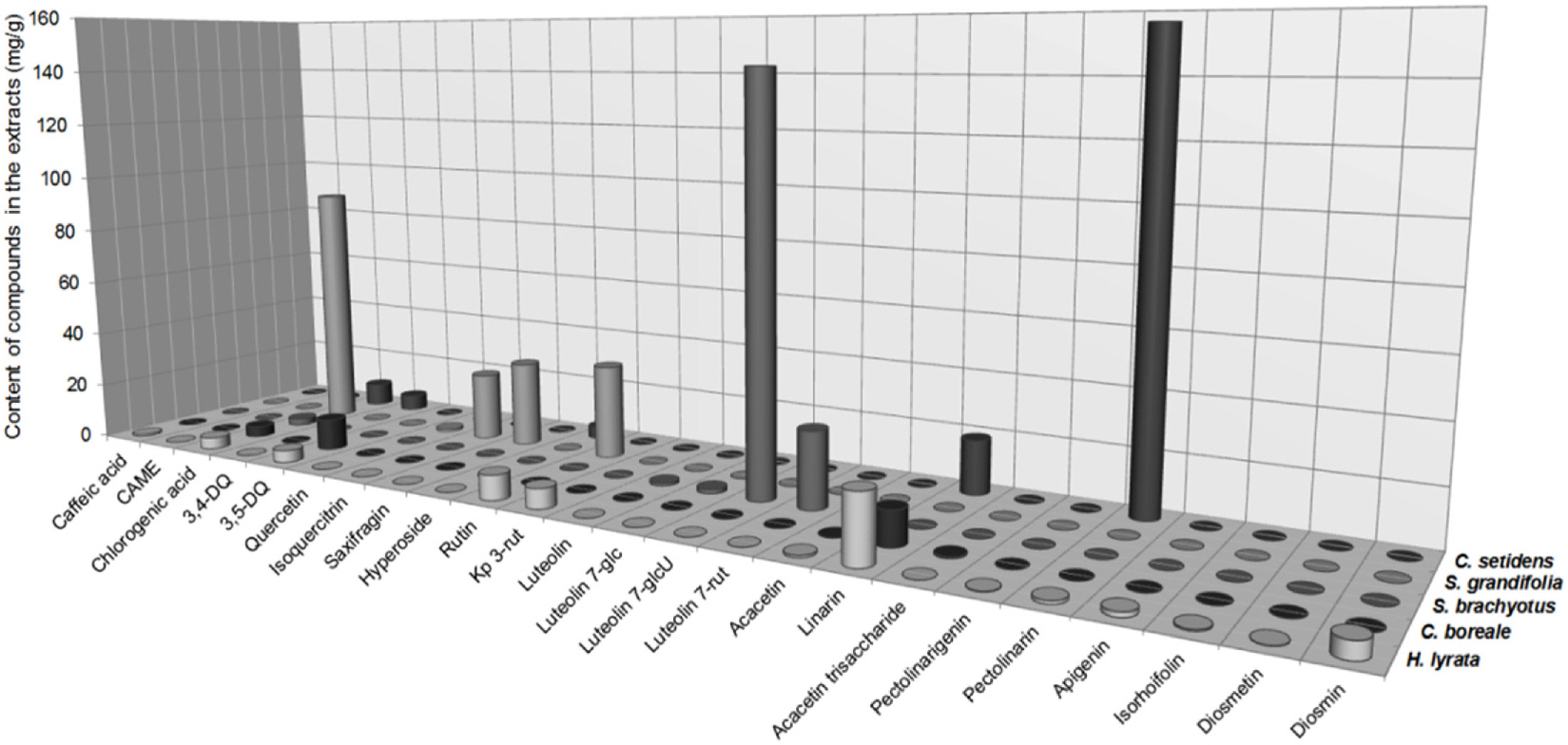
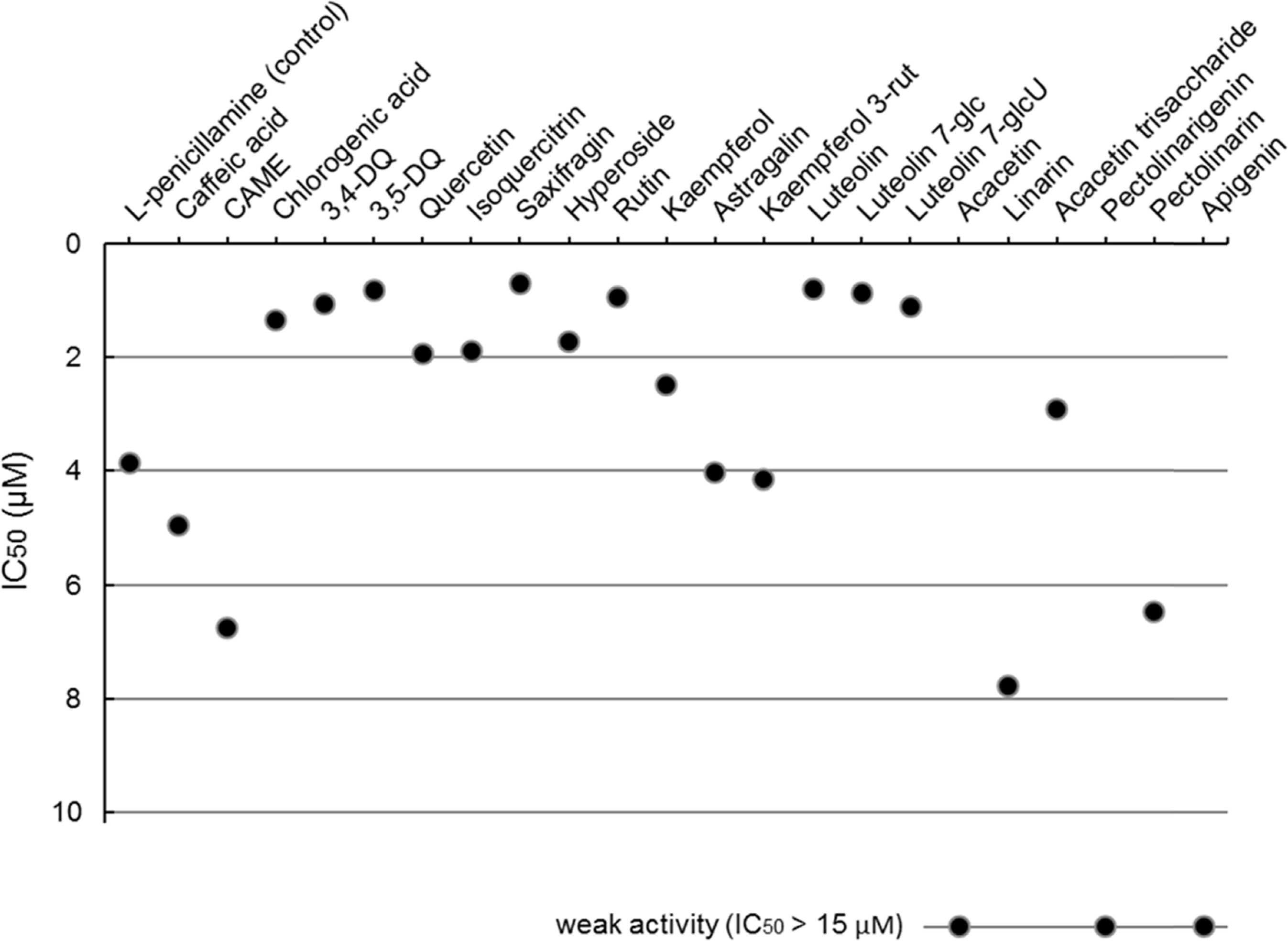
- Compositional differences in flavonoids are varied in the big family of Compositae. By summarizing our previous analytical studies and other scientific evidences, new strategy will be possible to further analyze flavonoids and utilize them for human health. The HPLC analytical method has been established in terms of linearity, sensitivity, accuracy, and precision. Herbs of the family of Compositae have considerable amounts of peroxynitrite (ONOO-)-scavenging effects and their phenolic substances. These effects may contribute to the prevention of disease associated with excess production of ONOO-, depending on the high content of flavonoid substances.
Keyword
MeSH Terms
Figure
Reference
-
(1). Fraisse D., Felgines C., Texier O., Lamaison J. L.Food Nutr. Sci. 2011; 2:181–192.(2). Nugroho A., Kim K. H., Lee K. R., Alam M. B., Choi J. S., Kim W. B., Park H. J.Arch. Pharm. Res. 2009; 32:1361–1367.(3). Nugroho A., Lee K. R., Alam M. B., Choi J. S., Park H. J.Arch. Pharm. Res. 2010; 33:703–708.(4). Pacher P., Beckman J. S., Liaudet L.Physiol. Rev. 2007; 87:315–424.(5). Nugroho A., Kim M. H., Lim S. C., Choi J. W., Choi J. S., Park H. J.Nat. Prod. Sci. 2011; 17:342–349.(6). Nugroho A., Lim S. C., Lee C. M., Choi J. S., Park H. J. J.Pharm. Biomed. Anal. 2012; 61:247–251.(7). Nugroho A., Kim M. H., Lee C. M., Choi J. S., Lee S. H., Park H. J.Nat. Prod. Sci. 2012; 18:39–46.(8). Nugroho A., Lim S. C., Choi J. W., Park H. J.Arch. Pharm. Res. 2013; 36:51–60.(9). Nugroho A., Lim S. C., Byeon J. S., Choi J. S., Park H. J. J.Pharm. Biomed. Anal. 2013; 76:139–144.(10). Schmidt R. J.Clin. Dermatol. 1986; 4:46–61.(11). Funk V. A., Susanna A., Stuessy T. F., Robinson H.Classifi-cation of Compositae. Systematics, evolution, and biogeography of the Compositae. Funk V.A., Susanna A., Bayer R. J., editorsInternational Association for Plant Taxonomy;Vienna: 2009. p. 174–189.(12). Konarev A. V., Anisimova I. N., Gavrilova V. A., Vachrusheva T. E., Konechnaya G. Y., Lewis M., Shewry P. R.Phytochemistry. 2002; 59:279–291.(13). Wegiera M., Smolarz H. D., Jedruch M., Korczak M., Kopro ní K.Acta Pol. Pharm. 2012; 69:263–268.(14). Jan G., Khan M. A., Jan F.Ethnobot. Leaflets. 2009; 13:1205–1215.(15). Ha T. J., Hwang S. W., Jung H. J., Park K. H., Yang M. S.Agric. Chem. Biotechnol. 2002; 45:170–172.(16). Hitmi A., Barthomeuf C., Coudret A. J.Plant Phys. 1998; 153:233–236.(17). Nicholson R. L., Hammerschmidt R.Annu. Rev. Phytopathol. 1992; 30:369–389.
Article(18). Ksouri R., Megdiche W., Falleh H., Trabelsi N., Boulaaba M., Smaoui A., Abdelly C. C. R.Biol. 2008; 331:865–873.(19). Han H., Baik B. K.Int. J. Food Sci. Tech. 2008; 43:1971–1978.
Article(20). Youdim K. A., Shukitt-Hale B., Joseph J. A.Free Radic. Biol. Med. 2004; 37:1683–1693.(21). Haenen G. R., Paquay J. B., Korthouwer R. E., Bast A.Biochem. Biophys. Res. Commun. 1997; 236:591–593.(22). Korda M., Kubant R., Patton S., Malinski T.Am. J. Physiol. Heart Circ. Physiol. 2008; 295:1514–1521.(23). Rayalam S., Della-Fera M. A., Baile C. A. J.Nutr. Biochem. 2008; 19:717–726.(24). Tórtora V., Quijano C., Freeman B., Radi R., Castro L.Free Radic. Biol. Med. 2007; 42:1075–1088.(25). Zhu S., Haddad I. Y., Matalon S.Arch. Biochem. Biophys. 1996; 333:282–290.(26). Radi R., Beckman J. S., Bush K. M., Freeman B.A.J. Biol. Chem. 1991; 266:4244–4250.(27). Tamura Y., Nakajima K., Nagayasu K., Takabayashi C.Phytochemistry. 2002; 59:275–278.(28). Velíšek J., Davídek J., Cejpek K.Czech J. Food Sci. 2008; 26:73–98.(29). Bowles D., Isayenkova J., Lim E. K., Poppenberger B.Curr. Opin. Plant Biol. 2005; 8:254–263.(30). Agati G., Biricolti S., Guidi L., Ferrini F., Fini A., Tattini M. J.Plant Physiol. 2011; 168:204–212.(31). Karki S., Park H. J., Nugroho A., Kim E. J., Jung H. A., Choi J. S. J.Med. Food. 2015; 18:83–94.(32). Nugroho A., Choi J. S., An H. J., Park H. J.Nat. Prod. Sci. 2015; 21:42–48.(33). Shimoi K., Okada H., Furugori M., Goda T., Takase S., Suzuki M., Hara Y., Yamamoto H., Kinae N.FEBS Lett. 1998; 438:220–224.(34). Schneider H., Blaut M.Arch. Microbiol. 2000; 173:71–75.(35). Lu J., Feng X., Sun Q., Lu H., Manabe M., Sugahara K., Ma D., Sagara Y., Kodama H.Clin. Chim. Acta. 2002; 316:95–99.(36). Hu C., Kitts D. D.Mol. Cell. Biochem. 2004; 265:107–113.(37). Jin M., Yang J. H., Lee E. K., Lu Y., Kwon S., Son K. H., Son J. K., Chang H. W.Biol. Pharm. Bull. 2009; 32:1500–1503.(38). Qiusheng Z., Xiling S., Xubo X. S., Meng S., Changhai W.Pharmazie. 2004; 59:286–289.(39). Vilela F. C., Soncini R., Giusti-Paiva A. J.Ethnopharmacol. 2009; 124:325–327.(40). Freitas C. S., Baggio C. H., Finau J., Anginoni M., Pizzolatti M. G., Santos A. R., Marques M. C. J.Pharm. Pharmacol. 2008; 60:1105–1110.(41). Kim J. S., Kwon C. S., Son K. H.Biosci. Biotechnol. Biochem. 2000; 64:2458–2461.(42). Han X. H., Hong S. S., Hwang J. S., Lee M. K., Hwang B. Y., Ro J. S.Arch. Pharm. Res. 2007; 30:13–17.(43). Brown J. E., Rice-Evans C. A.Free Radic. Res. 1998; 29:247–255.(44). Min Y. S., Bai K. L., Yim S. H., Lee Y. J., Song H. J., Kim J. H., Ham I. H., Whang W. K., Sohn U. D.Arch. Pharm. Res. 2006; 29:484–489.(45). Nagy M., Krizková L., Mucaji P., Kontseková Z., Sersen F., Krajcovic J.Molecules. 2009; 14:509–518.(46). Vilela F. C., Padilha-Mde M., Alves-da-Silva G., Soncini R., Giusti-Paiva A. J.Med. Food. 2010; 13:219–222.(47). Choi S. M., Kim B. C., Cho Y. H., Choi K. H., Chang J., Park M. S., Kim M. K., Cho K. H., Kim J. K.Chonnam Med. J. 2014; 50:45–51.(48). Salqueiro J. B., Ardenghi P., Dias M., Ferreira M. B. C., Izquierdo I., Medina J. H.Pharmacol. Biochem. Behav. 1997; 58:887–891.(49). Patil S. P., Jain P. D., Sancheti J. S., Ghumatkar P. J., Tambe R., Sathaye S.Neuropharmacolgy. 2014; 86:192–202.(50). Ha S. K., Moon E., Lee P., Ryu J. H., Oh M. S., Kim S. Y.Neurochem. Res. 2012; 37:1560–1567.(51). Watanabe K., Kanno S., Tomizawa A., Yomogida S., Ishikawa M.Oncol. Rep. 2012; 27:204–209.(52). Fan S. Y., Zeng H. W., Pei Y. H., Li L., Ye J., Pan Y. X., Zhang J. G., Yuan X., Zhang W. D. J.Ethnopharmacol. 2012; 141:647–652.(53). Calderone V., Chericoni S., Martinelli C., Testai L., Nardi A., Morelli I., Breschi M. C., Martinotti E.Naunyn Schmiedebergs Arch. Pharmacol. 2004; 370:290–298.(54). Kim H. R., Park C. G., Jung J. Y.Int. J. Mol. Med. 2014; 33:317–324.(55). Lim H., Son K. H., Chang H. W., Bae K., Kang S. S., Kim H. P.Biol. Pharm. Bull. 2008; 31:2063–2067.(56). Yoo Y. M., Nam J. H., Kim M. Y., Choi J., Park H. J.Biol Pharm Bull. 2008; 31:760–764.(57). Juckmeta T., Thongdeeying P., Itharat A.Evid. Based Complement. Alternat. Med. 2014; 2014:828760.(58). Bors W., Heller W., Michael C., Saran M.Adv. Exp. Med. Biol. 1990; 264:165–170.(59). Quiñones M., Miguel M., Aleixandre A.Pharmacol. Res. 2013; 68:125–131.(60). Iwai K., Kishimoto N., Kakino Y., Mochida K., Fujita T. J.Agric. Food Chem. 2004; 52:4893–4898.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Revisit to the Safety of Medicinal Herb
- A Study on Health Service Utilization and it's Determinants in the Low Income Family in Korea
- Quantitative Determination of Five Phenolic Peroxynitrite-scavengers in Nine Korean Native Compositae herbs
- A Study on Health and Public Health Center Utilization Behavior for lower Income Family in Korea
- The effects of insurance coverage on the medical care utilization in public health institutions in a rural area