Yonsei Med J.
2015 Jul;56(4):951-960. 10.3349/ymj.2015.56.4.951.
The Effects of a High Fat Diet Containing Diacylglycerol on Bone in C57BL/6J Mice
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Dongguk University Ilsan Hospital, Goyang, Korea.
- 2Division of Rheumatology, Department of Internal Medicine, Hallym University Sacred Heart Hospital, Anyang, Korea.
- 3Department of Cell and Developmental Biology, School of Dentistry, Seoul National University, Seoul, Korea.
- 4Division of Endocrinology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. lsk@yuhs.ac
- KMID: 2366334
- DOI: http://doi.org/10.3349/ymj.2015.56.4.951
Abstract
- PURPOSE
In epidemiologic and animal studies, a high fat diet (HFD) has been shown to be associated with lower bone mineral density (BMD) and a higher risk of osteoporotic fractures. Meanwhile, consuming a HFD containing diacylglycerol (DAG) instead of triacylglycerol (TAG) is known to offer metabolically beneficial effects of reductions in body weight and abdominal fat. The purpose of this study was to investigate the effects of a HFD containing DAG (HFD-DAG) on bone in mice.
MATERIALS AND METHODS
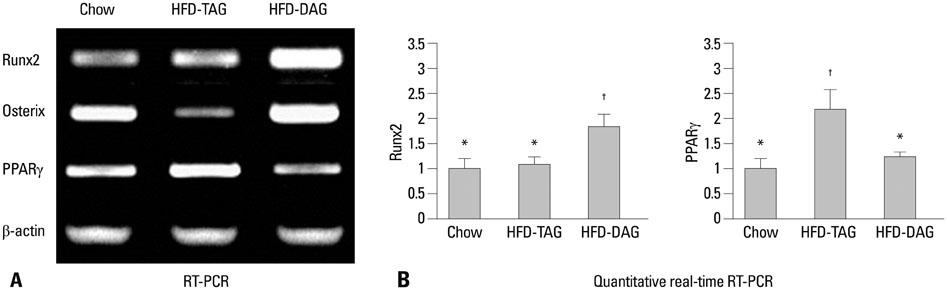
Four-week-old male C57BL/6J mice (n=39) were divided into three weight-matched groups based on diet type: a chow diet group, a HFD containing TAG (HFD-TAG) group, and a HFD-DAG group. After 20 weeks, body composition and bone microstructure were analyzed using dual energy X-ray absorptiometry and micro-computed tomography. Reverse transcription-polymerase chain reaction (PCR) and real-time PCR of bone marrow cells were performed to investigate the expressions of transcription factors for osteogenesis or adipogenesis.
RESULTS
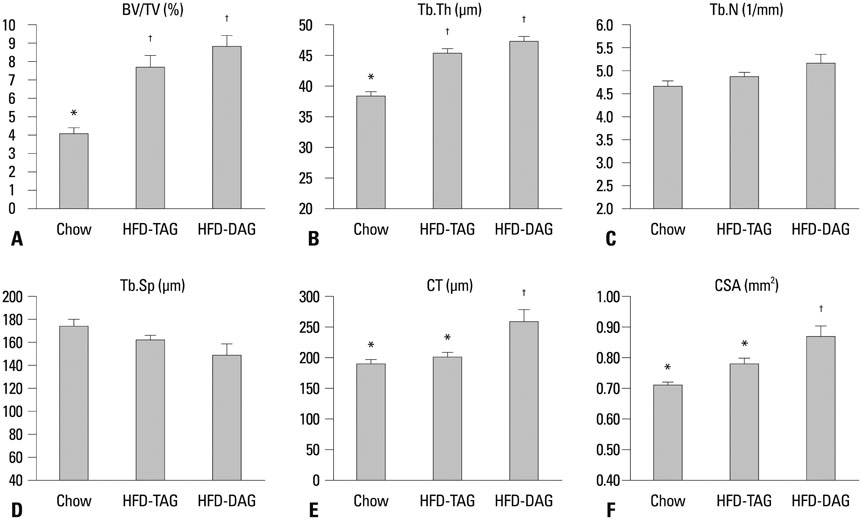
The HFD-DAG group exhibited lower body weight, higher BMD, and superior microstructural bone parameters, compared to the HFD-TAG group. The HFD-DAG group showed increased expression of Runx2 and decreased expression of PPARgamma in bone marrow cells, compared to the HFD-TAG group. The HFD-DAG group also had lower levels of plasma glucose, insulin, total cholesterol, and triglyceride than the HFD-TAG group.
CONCLUSION
Compared to HFD-TAG, HFD-DAG showed beneficial effects on bone and bone metabolism in C57BL/6J mice.
Keyword
MeSH Terms
-
Absorptiometry, Photon
Adipogenesis
Animals
Body Composition
Body Weight
Bone Density/*drug effects
Bone Marrow Cells/metabolism
Diet, High-Fat/*adverse effects
Dietary Fats/*pharmacology
Diglycerides/administration & dosage/*adverse effects
Male
Mice
Mice, Inbred C57BL
Osteogenesis/*drug effects
Real-Time Polymerase Chain Reaction
Triglycerides
X-Ray Microtomography
Dietary Fats
Diglycerides
Triglycerides
Figure
Reference
-
1. Lichtenstein AH, Kennedy E, Barrier P, Danford D, Ernst ND, Grundy SM, et al. Dietary fat consumption and health. Nutr Rev. 1998; 56(5 Pt 2):S3–19.
Article2. Kato I, Toniolo P, Zeleniuch-Jacquotte A, Shore RE, Koenig KL, Akhmedkhanov A, et al. Diet, smoking and anthropometric indices and postmenopausal bone fractures: a prospective study. Int J Epidemiol. 2000; 29:85–92.
Article3. Corwin RL, Hartman TJ, Maczuga SA, Graubard BI. Dietary saturated fat intake is inversely associated with bone density in humans: analysis of NHANES III. J Nutr. 2006; 136:159–165.
Article4. Parhami F, Tintut Y, Beamer WG, Gharavi N, Goodman W, Demer LL. Atherogenic high-fat diet reduces bone mineralization in mice. J Bone Miner Res. 2001; 16:182–188.
Article5. Bielohuby M, Matsuura M, Herbach N, Kienzle E, Slawik M, Hoeflich A, et al. Short-term exposure to low-carbohydrate, high-fat diets induces low bone mineral density and reduces bone formation in rats. J Bone Miner Res. 2010; 25:275–284.
Article6. Cao JJ, Gregoire BR, Gao H. High-fat diet decreases cancellous bone mass but has no effect on cortical bone mass in the tibia in mice. Bone. 2009; 44:1097–1104.
Article7. Xiao Y, Cui J, Li YX, Shi YH, Wang B, Le GW, et al. Dyslipidemic high-fat diet affects adversely bone metabolism in mice associated with impaired antioxidant capacity. Nutrition. 2011; 27:214–220.
Article8. Choi HS, Kim KJ, Kim KM, Hur NW, Rhee Y, Han DS, et al. Relationship between visceral adiposity and bone mineral density in Korean adults. Calcif Tissue Int. 2010; 87:218–225.
Article9. Hsu YH, Venners SA, Terwedow HA, Feng Y, Niu T, Li Z, et al. Relation of body composition, fat mass, and serum lipids to osteoporotic fractures and bone mineral density in Chinese men and women. Am J Clin Nutr. 2006; 83:146–154.
Article10. Parhami F. Possible role of oxidized lipids in osteoporosis: could hyperlipidemia be a risk factor? Prostaglandins Leukot Essent Fatty Acids. 2003; 68:373–378.
Article11. Basu S, Michaëlsson K, Olofsson H, Johansson S, Melhus H. Association between oxidative stress and bone mineral density. Biochem Biophys Res Commun. 2001; 288:275–279.
Article12. Baek KH, Oh KW, Lee WY, Lee SS, Kim MK, Kwon HS, et al. Association of oxidative stress with postmenopausal osteoporosis and the effects of hydrogen peroxide on osteoclast formation in human bone marrow cell cultures. Calcif Tissue Int. 2010; 87:226–235.
Article13. Flickinger BD, Matsuo N. Nutritional characteristics of DAG oil. Lipids. 2003; 38:129–132.
Article14. Rudkowska I, Roynette CE, Demonty I, Vanstone CA, Jew S, Jones PJ. Diacylglycerol: efficacy and mechanism of action of an anti-obesity agent. Obes Res. 2005; 13:1864–1876.
Article15. Yanai H, Tomono Y, Ito K, Furutani N, Yoshida H, Tada N. Diacylglycerol oil for the metabolic syndrome. Nutr J. 2007; 6:43.
Article16. Murase T, Mizuno T, Omachi T, Onizawa K, Komine Y, Kondo H, et al. Dietary diacylglycerol suppresses high fat and high sucrose diet-induced body fat accumulation in C57BL/6J mice. J Lipid Res. 2001; 42:372–378.
Article17. Murase T, Aoki M, Wakisaka T, Hase T, Tokimitsu I. Anti-obesity effect of dietary diacylglycerol in C57BL/6J mice: dietary diacylglycerol stimulates intestinal lipid metabolism. J Lipid Res. 2002; 43:1312–1319.
Article18. Hara K, Onizawa K, Honda H, Otsuji K, Ide T, Murata M. Dietary diacylglycerol-dependent reduction in serum triacylglycerol concentration in rats. Ann Nutr Metab. 1993; 37:185–191.
Article19. Taguchi H, Omachi T, Nagao T, Matsuo N, Tokimitsu I, Itakura H. Dietary diacylglycerol suppresses high fat diet-induced hepatic fat accumulation and microsomal triacylglycerol transfer protein activity in rats. J Nutr Biochem. 2002; 13:678–683.
Article20. Meng X, Zou D, Shi Z, Duan Z, Mao Z. Dietary diacylglycerol prevents high-fat diet-induced lipid accumulation in rat liver and abdominal adipose tissue. Lipids. 2004; 39:37–41.
Article21. Yanagisawa Y, Kawabata T, Tanaka O, Kawakami M, Hasegawa K, Kagawa Y. Improvement in blood lipid levels by dietary sn-1,3-diacylglycerol in young women with variants of lipid transporters 54T-FABP2 and -493g-MTP. Biochem Biophys Res Commun. 2003; 302:743–750.
Article22. Nagao T, Watanabe H, Goto N, Onizawa K, Taguchi H, Matsuo N, et al. Dietary diacylglycerol suppresses accumulation of body fat compared to triacylglycerol in men in a double-blind controlled trial. J Nutr. 2000; 130:792–797.
Article23. Maki KC, Davidson MH, Tsushima R, Matsuo N, Tokimitsu I, Umporowicz DM, et al. Consumption of diacylglycerol oil as part of a reduced-energy diet enhances loss of body weight and fat in comparison with consumption of a triacylglycerol control oil. Am J Clin Nutr. 2002; 76:1230–1236.
Article24. Taguchi H, Watanabe H, Onizawa K, Nagao T, Gotoh N, Yasukawa T, et al. Double-blind controlled study on the effects of dietary diacylglycerol on postprandial serum and chylomicron triacylglycerol responses in healthy humans. J Am Coll Nutr. 2000; 19:789–796.
Article25. Yamamoto K, Asakawa H, Tokunaga K, Watanabe H, Matsuo N, Tokimitsu I, et al. Long-term ingestion of dietary diacylglycerol lowers serum triacylglycerol in type II diabetic patients with hypertriglyceridemia. J Nutr. 2001; 131:3204–3207.
Article26. Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993; 123:1939–1951.
Article27. Kameda T, Mano H, Yuasa T, Mori Y, Miyazawa K, Shiokawa M, et al. Estrogen inhibits bone resorption by directly inducing apoptosis of the bone-resorbing osteoclasts. J Exp Med. 1997; 186:489–495.
Article28. Maurin AC, Chavassieux PM, Frappart L, Delmas PD, Serre CM, Meunier PJ. Influence of mature adipocytes on osteoblast proliferation in human primary cocultures. Bone. 2000; 26:485–489.
Article29. Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000; 100:197–207.
Article30. Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002; 111:305–317.
Article31. Thomas T, Gori F, Khosla S, Jensen MD, Burguera B, Riggs BL. Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology. 1999; 140:1630–1638.
Article32. Steppan CM, Crawford DT, Chidsey-Frink KL, Ke H, Swick AG. Leptin is a potent stimulator of bone growth in ob/ob mice. Regul Pept. 2000; 92:73–78.
Article33. Burguera B, Hofbauer LC, Thomas T, Gori F, Evans GL, Khosla S, et al. Leptin reduces ovariectomy-induced bone loss in rats. Endocrinology. 2001; 142:3546–3553.
Article34. Shinoda Y, Yamaguchi M, Ogata N, Akune T, Kubota N, Yamauchi T, et al. Regulation of bone formation by adiponectin through autocrine/paracrine and endocrine pathways. J Cell Biochem. 2006; 99:196–208.
Article35. Slim RM, Toborek M, Watkins BA, Boissonneault GA, Hennig B. Susceptibility to hepatic oxidative stress in rabbits fed different animal and plant fats. J Am Coll Nutr. 1996; 15:289–294.
Article36. Sreekumar R, Unnikrishnan J, Fu A, Nygren J, Short KR, Schimke J, et al. Impact of high-fat diet and antioxidant supplement on mitochondrial functions and gene transcripts in rat muscle. Am J Physiol Endocrinol Metab. 2002; 282:E1055–E1061.37. Mangiafico RA, Malaponte G, Pennisi P, Li Volti G, Trovato G, Mangiafico M, et al. Increased formation of 8-iso-prostaglandin F(2alpha) is associated with altered bone metabolism and lower bone mass in hypercholesterolaemic subjects. J Intern Med. 2007; 261:587–596.
Article38. Ostman B, Michaëlsson K, Helmersson J, Byberg L, Gedeborg R, Melhus H, et al. Oxidative stress and bone mineral density in elderly men: antioxidant activity of alpha-tocopherol. Free Radic Biol Med. 2009; 47:668–673.
Article39. Mody N, Parhami F, Sarafian TA, Demer LL. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic Biol Med. 2001; 31:509–519.
Article40. Bai XC, Lu D, Bai J, Zheng H, Ke ZY, Li XM, et al. Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-kappaB. Biochem Biophys Res Commun. 2004; 314:197–207.
Article41. Garrett IR, Boyce BF, Oreffo RO, Bonewald L, Poser J, Mundy GR. Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Invest. 1990; 85:632–639.
Article42. Lean JM, Jagger CJ, Kirstein B, Fuller K, Chambers TJ. Hydrogen peroxide is essential for estrogen-deficiency bone loss and osteoclast formation. Endocrinology. 2005; 146:728–735.
Article43. Parhami F, Morrow AD, Balucan J, Leitinger N, Watson AD, Tintut Y, et al. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler Thromb Vasc Biol. 1997; 17:680–687.
Article44. Parhami F, Jackson SM, Tintut Y, Le V, Balucan JP, Territo M, et al. Atherogenic diet and minimally oxidized low density lipoprotein inhibit osteogenic and promote adipogenic differentiation of marrow stromal cells. J Bone Miner Res. 1999; 14:2067–2078.
Article45. Tintut Y, Parhami F, Tsingotjidou A, Tetradis S, Territo M, Demer LL. 8-Isoprostaglandin E2 enhances receptor-activated NFkappa B ligand (RANKL)-dependent osteoclastic potential of marrow hematopoietic precursors via the cAMP pathway. J Biol Chem. 2002; 277:14221–14226.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of fermented blueberry liquid in high-fat diet-induced obese C57BL/6J mice
- Red beet (Beta vulgaris L.) leaf supplementation improves antioxidant status in C57BL/6J mice fed high fat high cholesterol diet
- Relationship between Bone Morphological Microstructure and Inflammatory Markers in Growing Mice Fed a High Fat Diet
- Grape seed extract (Vitis vinifera) partially reverses high fat diet-induced obesity in C57BL/6J mice
- Over-expression of myosin7A in cochlear hair cells of circling mice