Korean J Physiol Pharmacol.
2016 Nov;20(6):595-603. 10.4196/kjpp.2016.20.6.595.
The p90rsk-mediated signaling of ethanol-induced cell proliferation in HepG2 cell line
- Affiliations
-
- 1Department of Pharmacology, College of Pharmacy, Chung-Ang University, Seoul 06974, Korea. udsohn@cau.ac.kr
- 2Department of Medicinal Plant Science, College of Science and Engineering, Jungwon University, Chungbuk 28024, Korea.
- 3Department of Pharmacology, College of Pharmacy, Catholic University of Daegu, Daegu 38430, Korea.
- KMID: 2364263
- DOI: http://doi.org/10.4196/kjpp.2016.20.6.595
Abstract
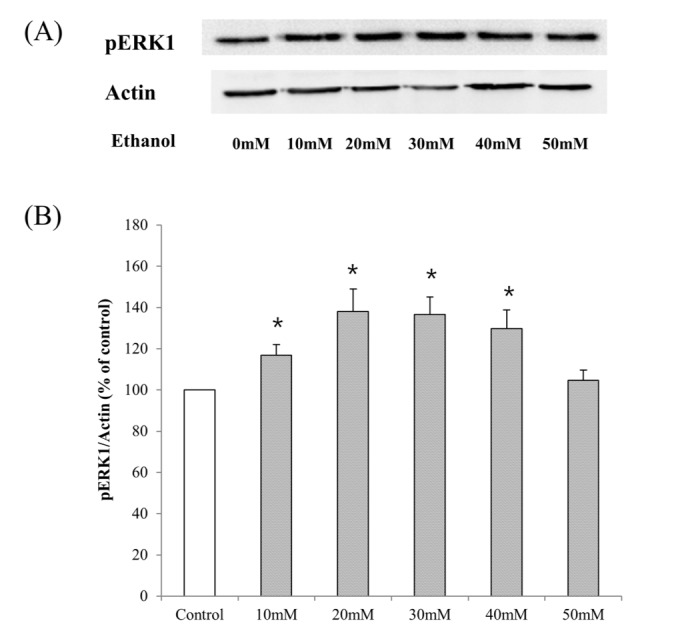
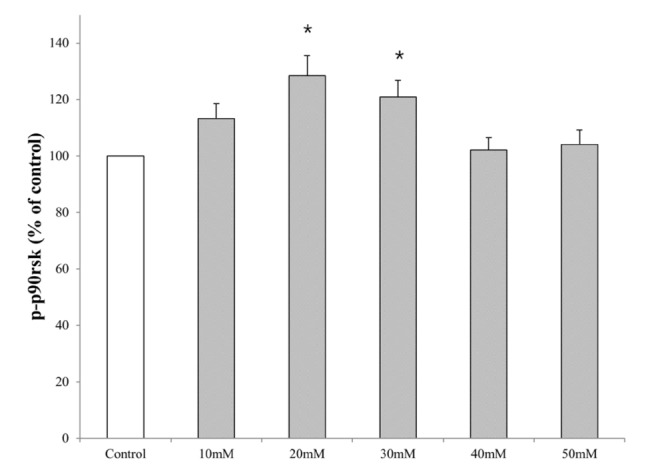
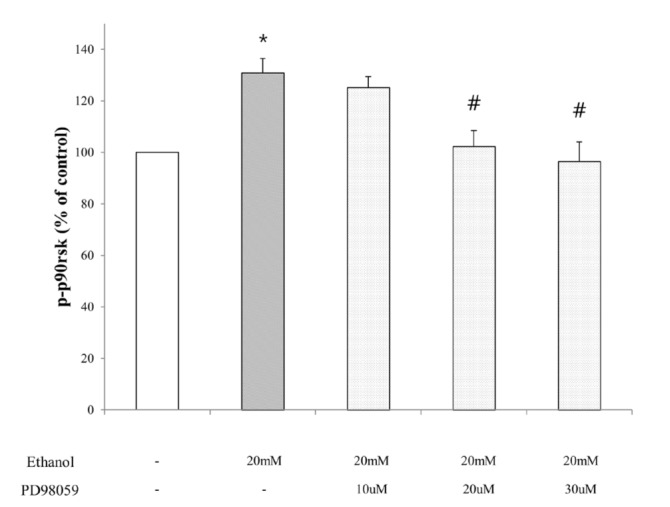
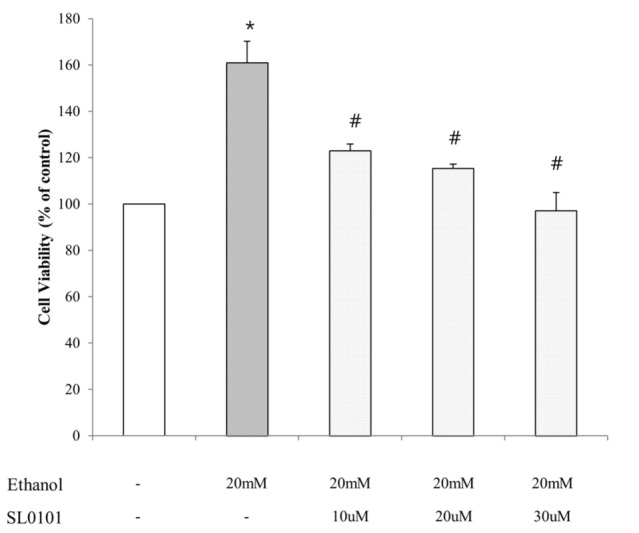
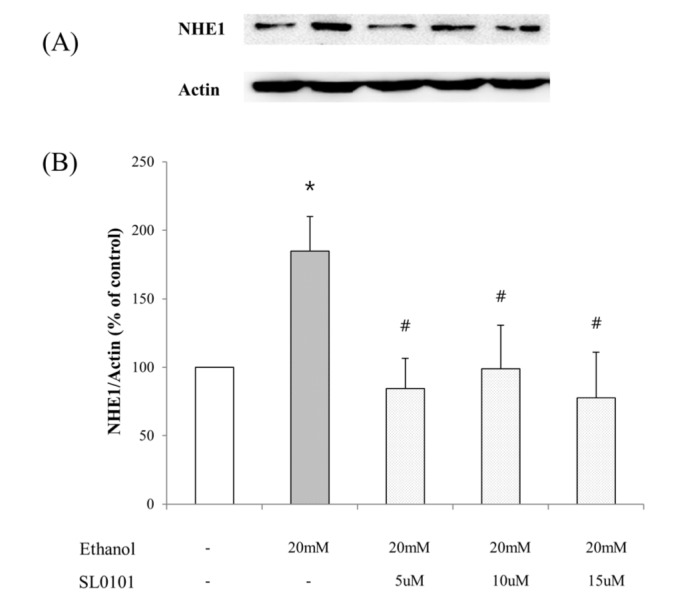
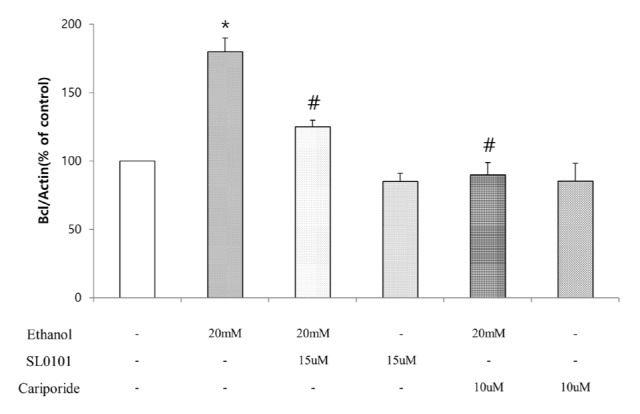
- Ribosomal S6 kinase is a family of serine/threonine protein kinases involved in the regulation of cell viability. There are two subfamilies of ribosomal s6 kinase, (p90rsk, p70rsk). Especially, p90rsk is known to be an important downstream kinase of p44/42 MAPK. We investigated the role of p90rsk on ethanol-induced cell proliferation of HepG2 cells. HepG2 cells were treated with 10~50 mM of ethanol with or without ERK and p90rsk inhibitors. Cell viability was measured by MTT assay. The expression of pERK1, NHE1 was measured by Western blots. The phosphorylation of p90rsk was measured by ELISA kits. The expression of Bcl-2 was measured by qRT-PCR. When the cells were treated with 10~30 mM of ethanol for 24 hour, it showed significant increase in cell viability versus control group. Besides, 10~30 mM of ethanol induced increased expression of pERK1, p-p90rsk, NHE1 and Bcl-2. Moreover treatment of p90rsk inhibitor attenuated the ethanol-induced increase in cell viability and NHE1 and Bcl-2 expression. In summary, these results suggest that p90rsk, a downstream kinase of ERK, plays a stimulatory role on ethanol-induced hepatocellular carcinoma progression by activating anti-apoptotic factor Bcl-2 and NHE1 known to regulate cell survival.
Keyword
MeSH Terms
Figure
Reference
-
1. Smith JA, Poteet-Smith CE, Xu Y, Errington TM, Hecht SM, Lannigan DA. Identification of the first specific inhibitor of p90 ribosomal S6 kinase (RSK) reveals an unexpected role for RSK in cancer cell proliferation. Cancer Res. 2005; 65:1027–1034. PMID: 15705904.2. Frödin M, Gammeltoft S. Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Mol Cell Endocrinol. 1999; 151:65–77. PMID: 10411321.
Article3. Roux PP, Richards SA, Blenis J. Phosphorylation of p90 ribosomal S6 kinase (RSK) regulates extracellular signal-regulated kinase docking and RSK activity. Mol Cell Biol. 2003; 23:4796–4804. PMID: 12832467.
Article4. Zhang Y, Zhong S, Dong Z, Chen N, Bode AM, Ma W, Dong Z. UVA induces Ser381 phosphorylation of p90RSK/MAPKAP-K1 via ERK and JNK pathways. J Biol Chem. 2001; 276:14572–14580. PMID: 11278279.
Article5. Clark DE, Errington TM, Smith JA, Frierson HF Jr, Weber MJ, Lannigan DA. The serine/threonine protein kinase, p90 ribosomal S6 kinase, is an important regulator of prostate cancer cell proliferation. Cancer Res. 2005; 65:3108–3116. PMID: 15833840.
Article6. Nguyen TT, Scimeca JC, Filloux C, Peraldi P, Carpentier JL, Van Obberghen E. Co-regulation of the mitogen-activated protein kinase, extracellular signal-regulated kinase 1, and the 90-kDa ribosomal S6 kinase in PC12 cells. Distinct effects of the neurotrophic factor, nerve growth factor, and the mitogenic factor, epidermal growth factor. J Biol Chem. 1993; 268:9803–9810. PMID: 8387505.
Article7. Chen RH, Blenis J. Identification of Xenopus S6 protein kinase homologs (pp90rsk) in somatic cells: phosphorylation and activation during initiation of cell proliferation. Mol Cell Biol. 1990; 10:3204–3215. PMID: 2342472.
Article8. Blenis J. Signal transduction via the MAP kinases: proceed at your own RSK. Proc Natl Acad Sci U S A. 1993; 90:5889–5892. PMID: 8392180.
Article9. Kalab P, Kubiak JZ, Verlhac MH, Colledge WH, Maro B. Activation of p90rsk during meiotic maturation and first mitosis in mouse oocytes and eggs: MAP kinase-independent and -dependent activation. Development. 1996; 122:1957–1964. PMID: 8674434.
Article10. Gross SD, Schwab MS, Taieb FE, Lewellyn AL, Qian YW, Maller JL. The critical role of the MAP kinase pathway in meiosis II in Xenopus oocytes is mediated by p90(Rsk). Curr Biol. 2000; 10:430–438. PMID: 10801413.
Article11. Tan Y, Ruan H, Demeter MR, Comb MJ. p90(RSK) blocks bad-mediated cell death via a protein kinase C-dependent pathway. J Biol Chem. 1999; 274:34859–34867. PMID: 10574959.
Article12. Law JH, Habibi G, Hu K, Masoudi H, Wang MY, Stratford AL, Park E, Gee JM, Finlay P, Jones HE, Nicholson RI, Carboni J, Gottardis M, Pollak M, Dunn SE. Phosphorylated insulin-like growth factor-i/insulin receptor is present in all breast cancer subtypes and is related to poor survival. Cancer Res. 2008; 68:10238–10246. PMID: 19074892.
Article13. Zoubeidi A, Zardan A, Wiedmann RM, Locke J, Beraldi E, Fazli L, Gleave ME. Hsp27 promotes insulin-like growth factor-I survival signaling in prostate cancer via p90Rsk-dependent phosphorylation and inactivation of BAD. Cancer Res. 2010; 70:2307–2317. PMID: 20197463.
Article14. Lucien F, Brochu-Gaudreau K, Arsenault D, Harper K, Dubois CM. Hypoxia-induced invadopodia formation involves activation of NHE-1 by the p90 ribosomal S6 kinase (p90RSK). PLoS One. 2011; 6:e28851. PMID: 22216126.
Article15. Liu Z, Wang S, Zhou H, Yang Y, Zhang M. Na+/H+ exchanger mediates TNF-alpha-induced hepatocyte apoptosis via the calpain-dependent degradation of Bcl-xL. J Gastroenterol Hepatol. 2009; 24:879–885. PMID: 19220664.16. Gillies RJ. The tumour microenvironment: causes and consequences of hypoxia and acidity. Introduction. Novartis Found Symp. 2001; 240:1–6. PMID: 11727923.17. Segal MS, Beem E. Effect of pH, ionic charge, and osmolality on cytochrome c-mediated caspase-3 activity. Am J Physiol Cell Physiol. 2001; 281:C1196–C1204. PMID: 11546656.18. Matsuyama S, Llopis J, Deveraux QL, Tsien RY, Reed JC. Changes in intramitochondrial and cytosolic pH: early events that modulate caspase activation during apoptosis. Nat Cell Biol. 2000; 2:318–325. PMID: 10854321.
Article19. Putney LK, Denker SP, Barber DL. The changing face of the Na+/H+ exchanger, NHE1: structure, regulation, and cellular actions. Annu Rev Pharmacol Toxicol. 2002; 42:527–552. PMID: 11807182.20. Wu KL, Khan S, Lakhe-Reddy S, Wang L, Jarad G, Miller RT, Konieczkowski M, Brown AM, Sedor JR, Schelling JR. Renal tubular epithelial cell apoptosis is associated with caspase cleavage of the NHE1 Na+/H+ exchanger. Am J Physiol Renal Physiol. 2003; 284:F829–F839. PMID: 12453872.21. Li J, Eastman A. Apoptosis in an interleukin-2-dependent cytotoxic T lymphocyte cell line is associated with intracellular acidification. Role of the Na+/H+-antiport. J Biol Chem. 1995; 270:3203–3211. PMID: 7852405.22. Luo J, Tannock IF. Inhibition of the regulation of intracellular pH: potential of 5-(N,N-hexamethylene) amiloride in tumour-selective therapy. Br J Cancer. 1994; 70:617–624. PMID: 7917906.
Article23. Maidorn RP, Cragoe EJ Jr, Tannock IF. Therapeutic potential of analogues of amiloride: inhibition of the regulation of intracellular pH as a possible mechanism of tumour selective therapy. Br J Cancer. 1993; 67:297–303. PMID: 8381657.
Article24. Thangaraju M, Sharma K, Liu D, Shen SH, Srikant CB. Interdependent regulation of intracellular acidification and SHP-1 in apoptosis. Cancer Res. 1999; 59:1649–1654. PMID: 10197642.25. Shrode LD, Tapper H, Grinstein S. Role of intracellular pH in proliferation, transformation, and apoptosis. J Bioenerg Biomembr. 1997; 29:393–399. PMID: 9387100.26. Packham G. Mutation of BCL-2 family proteins in cancer. Apoptosis. 1998; 3:75–82. PMID: 14646504.27. Reynolds JE, Li J, Craig RW, Eastman A. BCL-2 and MCL-1 expression in Chinese hamster ovary cells inhibits intracellular acidification and apoptosis induced by staurosporine. Exp Cell Res. 1996; 225:430–436. PMID: 8660932.
Article28. Harguindey S, Pedraz JL, Cañero RG, Katin M. Edelfosine, apoptosis, MDR and Na+/H+ exchanger: induction mechanisms and treatment implications. Apoptosis. 2000; 5:87–89. PMID: 11227496.29. Lee KW, Kim SG, Kim HP, Kwon E, You J, Choi HJ, Park JH, Kang BC, Im SA, Kim TY, Kim WH, Bang YJ. Enzastaurin, a protein kinase C beta inhibitor, suppresses signaling through the ribosomal S6 kinase and bad pathways and induces apoptosis in human gastric cancer cells. Cancer Res. 2008; 68:1916–1926. PMID: 18339873.30. Meng XB, Sun GB, Wang M, Sun J, Qin M, Sun XB. P90RSK and Nrf2 activation via MEK1/2-ERK1/2 pathways mediated by Notoginsenoside R2 to prevent 6-hydroxydopamine-induced apoptotic death in SH-SY5Y cells. Evid Based Complement Alternat Med. 2013; DOI: 10.1155/2013/971712.
Article31. Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993; 74:609–619. PMID: 8358790.32. Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999; 13:1899–1911. PMID: 10444588.
Article33. Kubasiak LA, Hernandez OM, Bishopric NH, Webster KA. Hypoxia and acidosis activate cardiac myocyte death through the Bcl-2 family protein BNIP3. Proc Natl Acad Sci U S A. 2002; 99:12825–12830. PMID: 12226479.
Article34. Anjum R, Blenis J. The RSK family of kinases: emerging roles in cellular signalling. Nat Rev Mol Cell Biol. 2008; 9:747–758. PMID: 18813292.
Article35. Hennig M, Yip-Schneider MT, Klein P, Wentz S, Matos JM, Doyle C, Choi J, Wu H, O'Mara A, Menze A, Noble S, McKillop IH, Schmidt CM. Ethanol-TGFalpha-MEK signaling promotes growth of human hepatocellular carcinoma. J Surg Res. 2009; 154:187–195. PMID: 19321179.36. Smadja-Lamère N, Shum M, Déléris P, Roux PP, Abe J, Marette A. Insulin activates RSK (p90 ribosomal S6 kinase) to trigger a new negative feedback loop that regulates insulin signaling for glucose metabolism. J Biol Chem. 2013; 288:31165–31176. PMID: 24036112.37. Chen SJ, Karan D, Johansson SL, Lin FF, Zeckser J, Singh AP, Batra SK, Lin MF. Prostate-derived factor as a paracrine and autocrine factor for the proliferation of androgen receptor-positive human prostate cancer cells. Prostate. 2007; 67:557–571. PMID: 17221842.
Article38. Amorino GP, Hamilton VM, Valerie K, Dent P, Lammering G, Schmidt-Ullrich RK. Epidermal growth factor receptor dependence of radiation-induced transcription factor activation in human breast carcinoma cells. Mol Biol Cell. 2002; 13:2233–2244. PMID: 12134064.
Article39. Harguindey S, Orive G, Luis Pedraz J, Paradiso A, Reshkin SJ. The role of pH dynamics and the Na+/H+ antiporter in the etiopathogenesis and treatment of cancer. Two faces of the same coin--one single nature. Biochim Biophys Acta. 2005; 1756:1–24. PMID: 16099110.40. Reshkin SJ, Bellizzi A, Caldeira S, Albarani V, Malanchi I, Poignee M, Alunni-Fabbroni M, Casavola V, Tommasino M. Na+/H+ exchanger-dependent intracellular alkalinization is an early event in malignant transformation and plays an essential role in the development of subsequent transformation-associated phenotypes. FASEB J. 2000; 14:2185–2197. PMID: 11053239.41. Tan N, Wong M, Nannini MA, Hong R, Lee LB, Price S, Williams K, Savy PP, Yue P, Sampath D, Settleman J, Fairbrother WJ, Belmont LD. Bcl-2/Bcl-xL inhibition increases the efficacy of MEK inhibition alone and in combination with PI3 kinase inhibition in lung and pancreatic tumor models. Mol Cancer Ther. 2013; 12:853–864. PMID: 23475955.
Article42. Rajah TT, Peine KJ, Du N, Serret CA, Drews NR. Physiological concentrations of genistein and 17β-estradiol inhibit MDA-MB-231 breast cancer cell growth by increasing BAX/BCL-2 and reducing pERK1/2. Anticancer Res. 2012; 32:1181–1191. PMID: 22493348.43. Zhou J, Wulfkuhle J, Zhang H, Gu P, Yang Y, Deng J, Margolick JB, Liotta LA, Petricoin E 3rd, Zhang Y. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc Natl Acad Sci U S A. 2007; 104:16158–16163. PMID: 17911267.
Article44. Gwin J, Drews N, Ali S, Stamschror J, Sorenson M, Rajah TT. Effect of genistein on p90RSK phosphorylation and cell proliferation in T47D breast cancer cells. Anticancer Res. 2011; 31:209–214. PMID: 21273600.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of short-term ethanol on the proliferative response of Swiss 3T3 cells to mitogenic growth factors
- Molecular Targeting of ERKs/RSK2 Signaling Axis in Cancer Prevention
- Cell Beath Induced by Ethanol : Prevention of Cell Death by the bcl-2 Proto-Oncogene
- Differential Thioredoxin Reductase Activity from Human Normal Hepatic and Hepatoma Cell Lines
- Mitogenic Effects and Signaling Pathway of Insulin-Like Growth Factor-I (IGF-I) in the Rat Beta Cell Line (INS-1)