J Pathol Transl Med.
2016 Sep;50(5):369-376. 10.4132/jptm.2016.06.06.
Long Non-coding RNA HOTAIR Expression in Diffuse Large B-Cell Lymphoma: In Relation to Polycomb Repressive Complex Pathway Proteins and H3K27 Trimethylation
- Affiliations
-
- 1Department of Pathology, Yonsei University College of Medicine, Seoul, Korea. soyoon@yuhs.ac
- 2Department of Pathology, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 3Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 4Anatomic Pathology Reference Lab, Seegene Medical Foundation, Seoul, Korea.
- KMID: 2353599
- DOI: http://doi.org/10.4132/jptm.2016.06.06
Abstract
- BACKGROUND
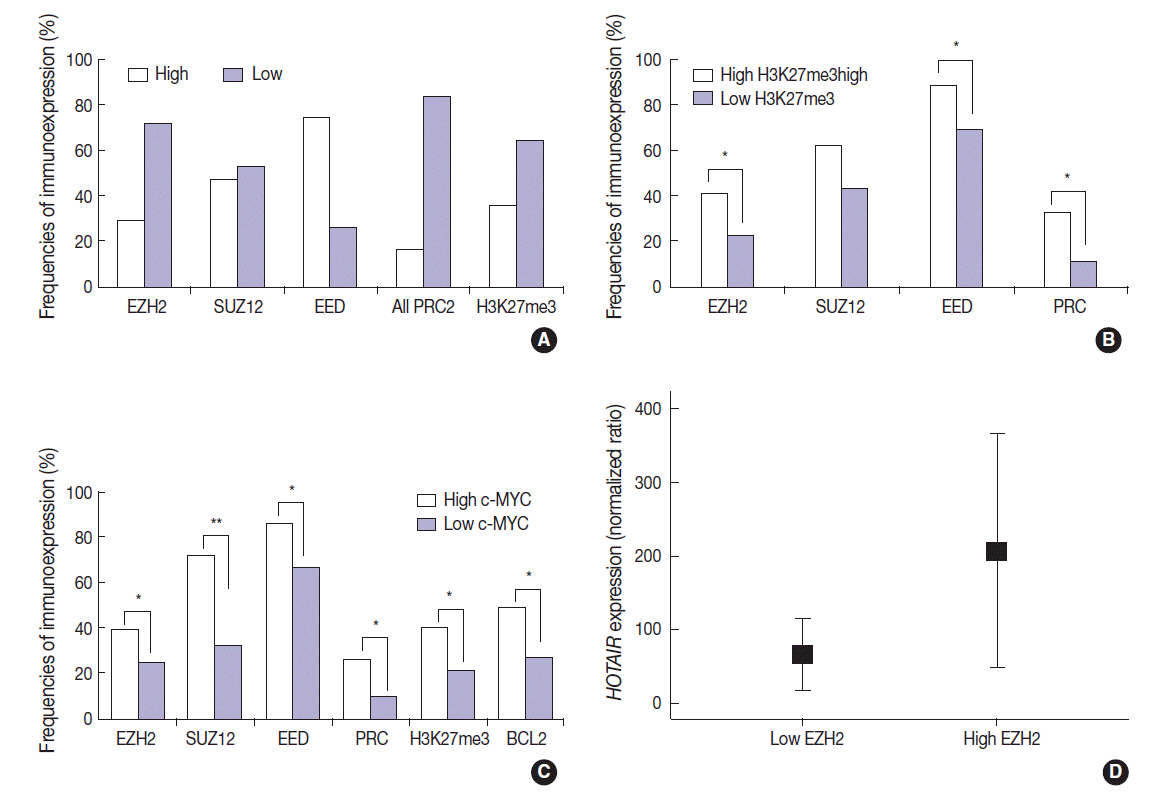
A long non-coding RNA hox transcript antisense intergenic RNA (HOTAIR) is involved in epigenetic regulation through chromatin remodeling by recruiting polycomb repressive complex 2 (PRC2) proteins (EZH2, SUZ12, and EED) that induce histone H3 trimethylation at lysine 27 (H3K27me3). Deregulation of c-MYC and interaction between c-MYC and EZH2 are well known in lymphomagenesis; however, little is known about the expression status of HOTAIR in diffuse large B-cell lymphomas (DLBCLs).
METHODS
The expression status of PRC2 (EZH2, SUZ12, and EED), H3K27me3, c-MYC, and BCL2 was analyzed using immunohistochemistry (n = 231), and HOTAIR was investigated by a quantification real-time polymerase chain reaction method (n = 164) in DLBCLs.
RESULTS
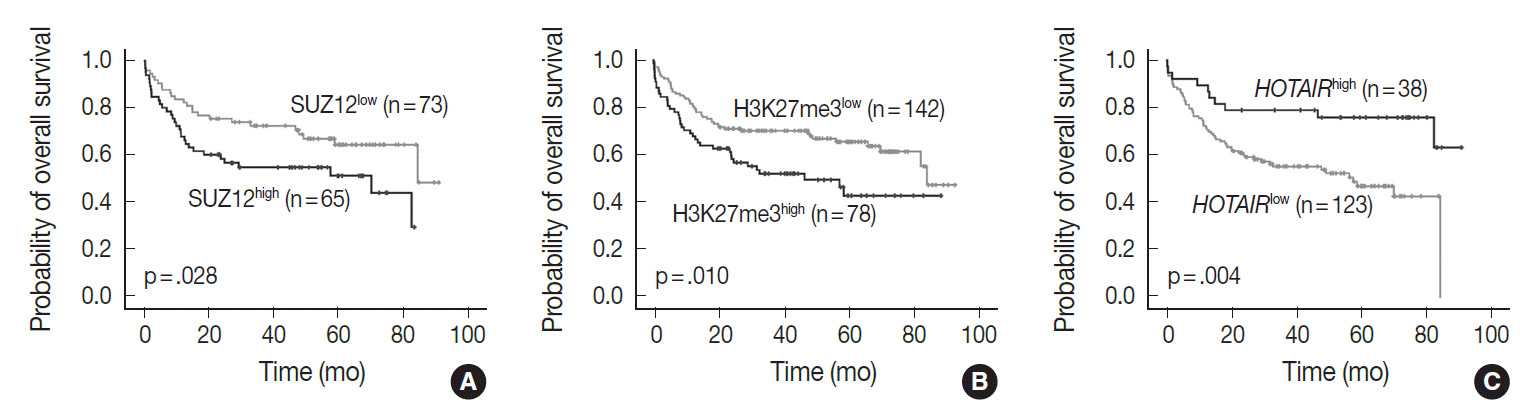
The present study confirmed the positive correlation among PRC2 proteins, H3K27me3, and c-MYC in DLBCLs. Expression level of HOTAIR was also positively correlated to EZH2 (p < .05, respectively). Between c-MYC and HOTAIR, and between c- MYC/BCL2 co-expression and HOTAIR, however, negative correlation was observed in DLBCLs (p < .05, respectively). High level of H3K27me3 was determined as an independent prognostic marker in poor overall survival (hazard ratio, 2.0; p = .023) of DLBCL patients. High expression of HOTAIR, however, was associated with favorable overall survival (p = .004) in the univariate analysis, but the impact was not significant in the multivariate analysis. The favorable outcome of DLBCL with HOTAIR high expression levels may be related to the negative correlation with c- MYC expression or c-MYC/BCL2 co-expression.
CONCLUSIONS
HOTAIR expression could be one of possible mechanisms for inducing H3K27me3 via EZH2-related PRC2 activation, and induced H3K27me3 may be strongly related to aggressive DLBCLs which show poor patient outcome.
Keyword
MeSH Terms
-
B-Lymphocytes*
Chromatin Assembly and Disassembly
Epigenomics
Histones
Humans
Immunohistochemistry
Lymphoma, B-Cell*
Lymphoma, Large B-Cell, Diffuse
Lysine
Methods
Multivariate Analysis
Polycomb Repressive Complex 2
Real-Time Polymerase Chain Reaction
RNA
RNA, Long Noncoding*
Histones
Lysine
Polycomb Repressive Complex 2
RNA
RNA, Long Noncoding
Figure
Reference
-
1. Spizzo R, Almeida MI, Colombatti A, Calin GA. Long non-coding RNAs and cancer: a new frontier of translational research? Oncogene. 2012; 31:4577–87.
Article2. Wahlestedt C. Targeting long non-coding RNA to therapeutically upregulate gene expression. Nat Rev Drug Discov. 2013; 12:433–46.
Article3. Gupta RA, Shah N, Wang KC, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010; 464:1071–6.4. Qiu MT, Hu JW, Yin R, Xu L. Long noncoding RNA: an emerging paradigm of cancer research. Tumour Biol. 2013; 34:613–20.
Article5. Zhang S, Chen S, Yang G, et al. Long noncoding RNA HOTAIR as an independent prognostic marker in cancer: a meta-analysis. PLoS One. 2014; 9:e105538.6. Martin-Perez D, Piris MA, Sanchez-Beato M. Polycomb proteins in hematologic malignancies. Blood. 2010; 116:5465–75.
Article7. Bracken AP, Helin K. Polycomb group proteins: navigators of lineage pathways led astray in cancer. Nat Rev Cancer. 2009; 9:773–84.
Article8. Oh EJ, Yang WI, Cheong JW, Choi SE, Yoon SO. Diffuse large B-cell lymphoma with histone H3 trimethylation at lysine 27: another poor prognostic phenotype independent of c-Myc/Bcl2 coexpression. Hum Pathol. 2014; 45:2043–50.
Article9. Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: IARC Press;2008.10. Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004; 103:275–82.
Article11. Johnson NA, Slack GW, Savage KJ, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012; 30:3452–9.12. Hu S, Xu-Monette ZY, Tzankov A, et al. MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL Rituximab-CHOP Consortium Program. Blood. 2013; 121:4021–31.
Article13. Benetatos L, Vartholomatos G, Hatzimichael E. Polycomb group proteins and MYC: the cancer connection. Cell Mol Life Sci. 2014; 71:257–69.
Article14. Sander S, Bullinger L, Klapproth K, et al. MYC stimulates EZH2 expression by repression of its negative regulator miR-26a. Blood. 2008; 112:4202–12.
Article15. Neri F, Zippo A, Krepelova A, Cherubini A, Rocchigiani M, Oliviero S. Myc regulates the transcription of the PRC2 gene to control the expression of developmental genes in embryonic stem cells. Mol Cell Biol. 2012; 32:840–51.16. McCabe MT, Ott HM, Ganji G, et al. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature. 2012; 492:108–12.
Article17. Qi W, Chan H, Teng L, et al. Selective inhibition of Ezh2 by a small molecule inhibitor blocks tumor cells proliferation. Proc Natl Acad Sci U S A. 2012; 109:21360–5.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- HOTAIR Long Non-coding RNA: Characterizing the Locus Features by the In Silico Approaches
- Regulation and Role of EZH2 in Cancer
- HOTAIR Induces Methylation of PCDH10, a Tumor Suppressor Gene, by Regulating DNMT1 and Sponging with miR-148b in Gastric Adenocarcinoma
- Circulating HOTAIR LncRNA Is Potentially Up-regulated in Coronary Artery Disease
- Genomics of diffuse large B cell lymphoma