Korean J Physiol Pharmacol.
2016 Sep;20(5):487-498. 10.4196/kjpp.2016.20.5.487.
p53 signaling is involved in leptin-induced growth of hepatic and breast cancer cells
- Affiliations
-
- 1College of Pharmacy, Yeungnam University, Gyeongsan 38541, Korea. parkp@yu.ac.kr
- KMID: 2350505
- DOI: http://doi.org/10.4196/kjpp.2016.20.5.487
Abstract
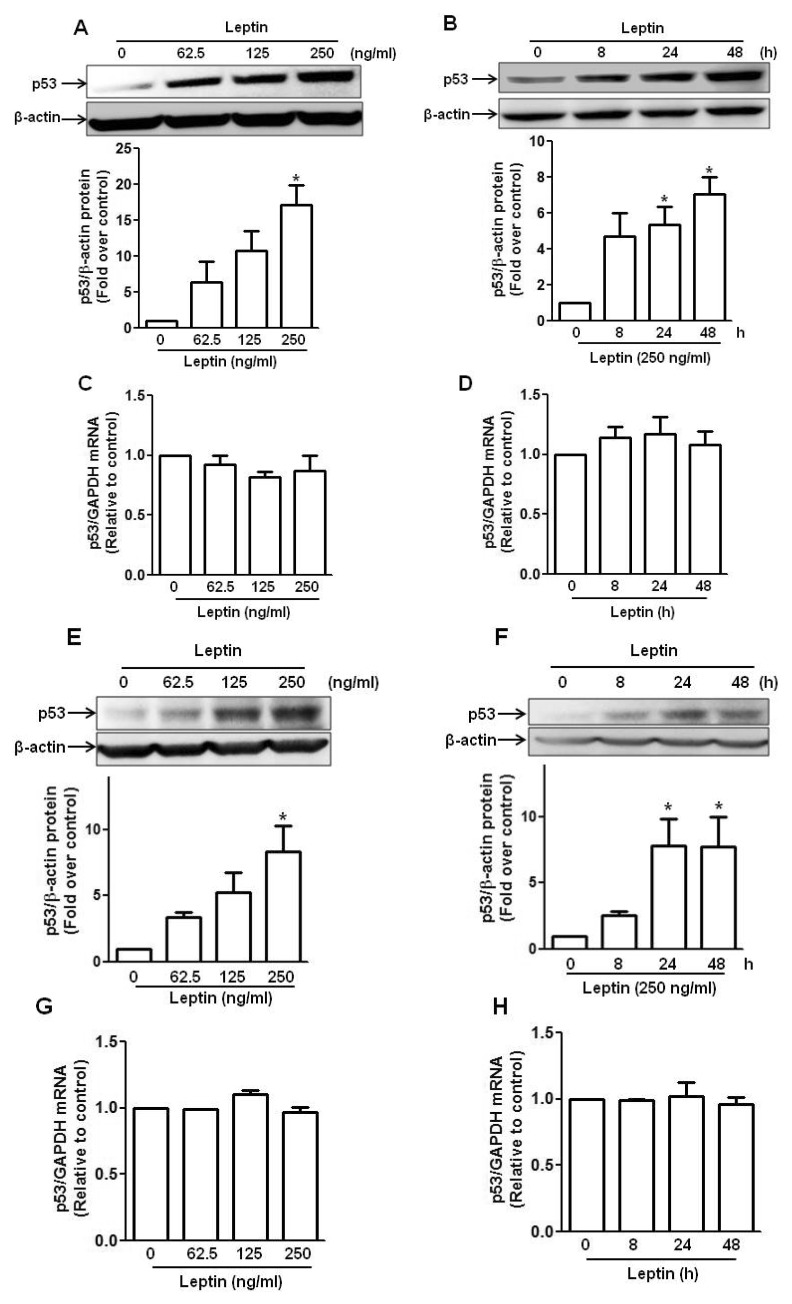
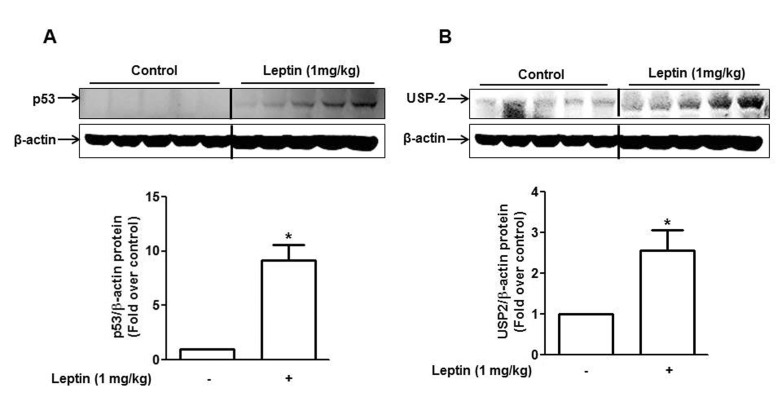
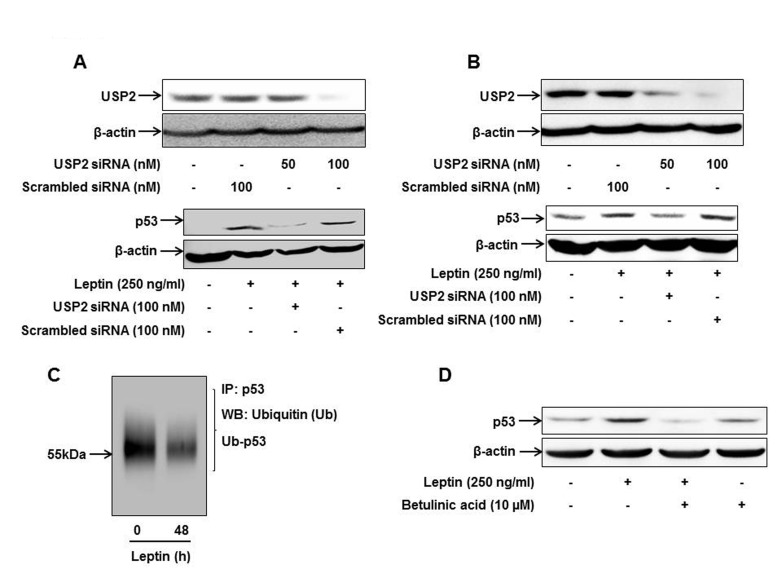
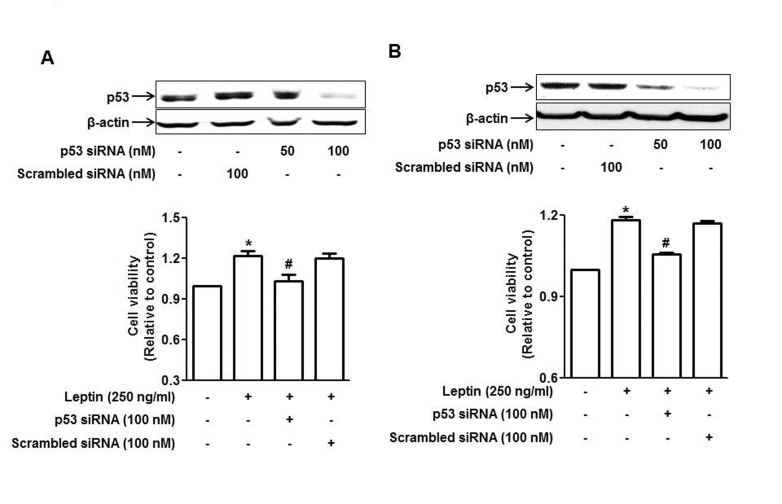
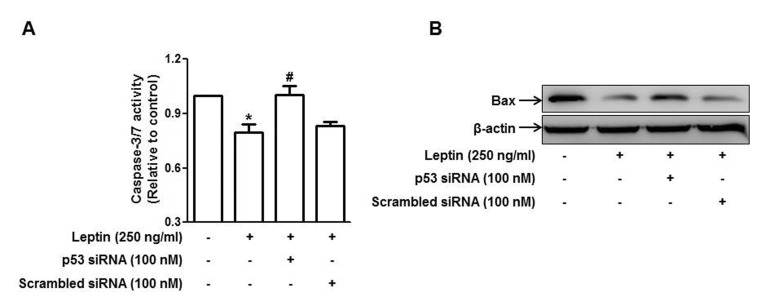
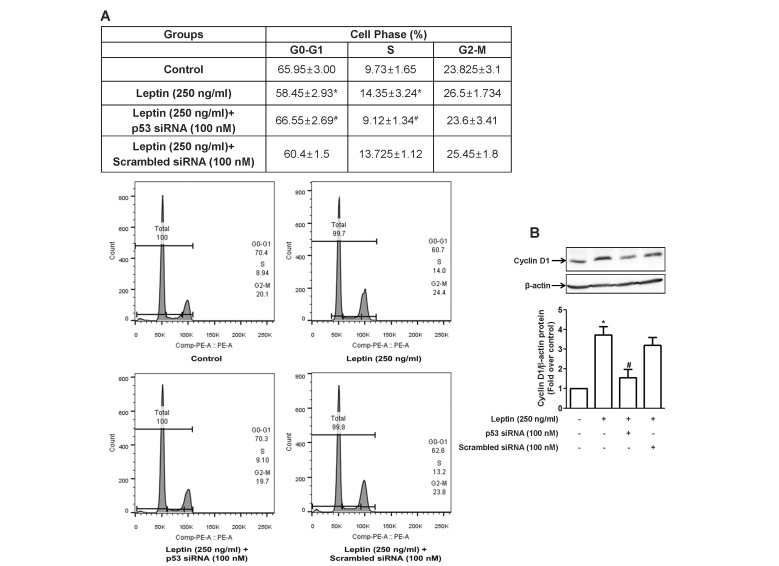
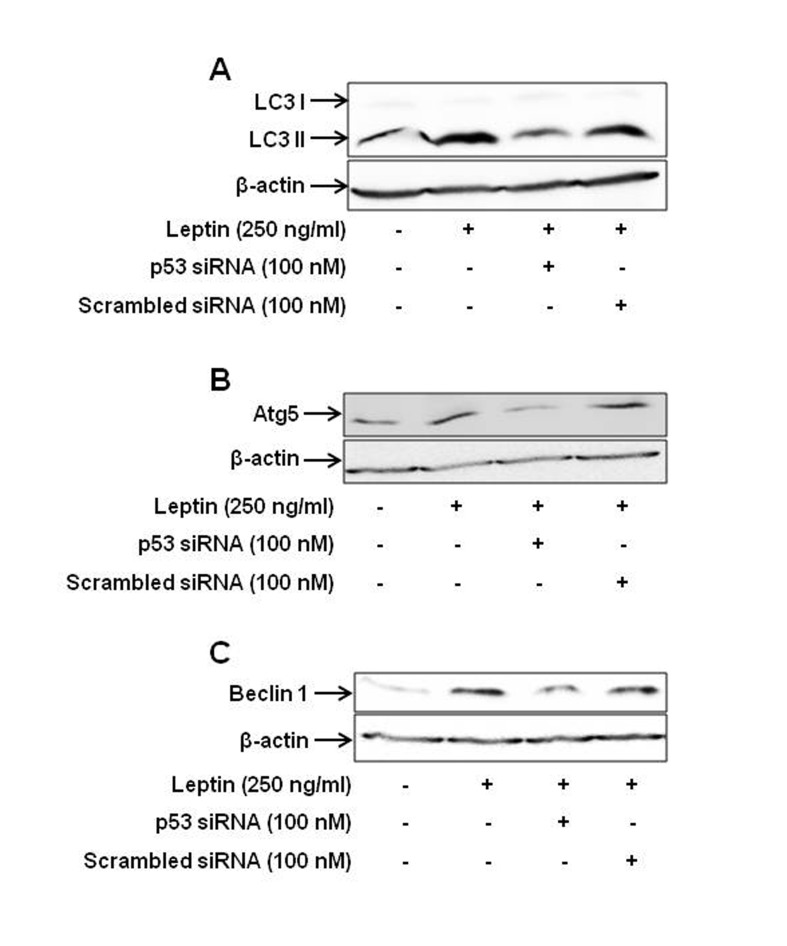
- Leptin, an adipokine predominantly produced from adipose tissue, is well known to induce tumor growth. However, underlying molecular mechanisms are not established yet. While p53 has long been well recognized as a potent tumor suppressor gene, accumulating evidence has also indicated its potential role in growth and survival of cancer cells depending on experimental environments. In the present study, we examined if p53 signaling is implicated in leptin-induced growth of cancer cells. Herein, we demonstrated that leptin treatment significantly increased p53 protein expression in both hepatic (HepG2) and breast (MCF-7) cancer cells without significant effect on mRNA expression. Enhanced p53 expression by leptin was mediated via modulation of ubiquitination, in particular ubiquitin specific protease 2 (USP2)-dependent manner. Furthermore, gene silencing of p53 by small interfering RNA (siRNA) suppressed leptin-induced growth of hepatic and breast cancer cells, indicating the role of p53 signaling in tumor growth by leptin. In addition, we also showed that knockdown of p53 restored suppression of caspase-3 activity by leptin through modulating Bax expression and prevented leptin-induced cell cycle progression, implying the involvement of p53 signaling in the regulation of both apoptosis and cell cycle progression in cancer cells treated with leptin. Taken together, the results in the present study demonstrated the potential role of p53 signaling in leptin-induced tumor growth.
Keyword
MeSH Terms
Figure
Reference
-
1. Myers MG, Cowley MA, Münzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2008; 70:537–556. PMID: 17937601.
Article2. Chen C, Chang YC, Liu CL, Liu TP, Chang KJ, Guo IC. Leptin induces proliferation and anti-apoptosis in human hepatocarcinoma cells by up-regulating cyclin D1 and down-regulating Bax via a Janus kinase 2-linked pathway. Endocr Relat Cancer. 2007; 14:513–529. PMID: 17639064.
Article3. Garofalo C, Surmacz E. Leptin and cancer. J Cell Physiol. 2006; 207:12–22. PMID: 16110483.
Article4. Perera CN, Chin HG, Duru N, Camarillo IG. Leptin-regulated gene expression in MCF-7 breast cancer cells: mechanistic insights into leptin-regulated mammary tumor growth and progression. J Endocrinol. 2008; 199:221–233. PMID: 18715880.
Article5. Andò S, Catalano S. The multifactorial role of leptin in driving the breast cancer microenvironment. Nat Rev Endocrinol. 2011; 8:263–275. PMID: 22083089.
Article6. Nepal S, Kim MJ, Hong JT, Kim SH, Sohn DH, Lee SH, Song K, Choi DY, Lee ES, Park PH. Autophagy induction by leptin contributes to suppression of apoptosis in cancer cells and xenograft model: involvement of p53/FoxO3A axis. Oncotarget. 2015; 6:7166–7181. PMID: 25704884.
Article7. Vousden KH, Lu X. Live or let die: the cell's response to p53. Nat Rev Cancer. 2002; 2:594–604. PMID: 12154352.
Article8. Liu J, Zhang C, Feng Z. Tumor suppressor p53 and its gain-offunction mutants in cancer. Acta Biochim Biophys Sin (Shanghai). 2014; 46:170–179. PMID: 24374774.
Article9. Yee KS, Vousden KH. Complicating the complexity of p53. Carcinogenesis. 2005; 26:1317–1322. PMID: 15888490.
Article10. Chipuk JE, Green DR. Dissecting p53-dependent apoptosis. Cell Death Differ. 2006; 13:994–1002. PMID: 16543937.
Article11. Scherz-Shouval R, Weidberg H, Gonen C, Wilder S, Elazar Z, Oren M. p53-dependent regulation of autophagy protein LC3 supports cancer cell survival under prolonged starvation. Proc Natl Acad Sci U S A. 2010; 107:18511–18516. PMID: 20937856.
Article12. Lo PK, Huang SZ, Chen HC, Wang FF. The prosurvival activity of p53 protects cells from UV-induced apoptosis by inhibiting c-Jun NH2-terminal kinase activity and mitochondrial death signaling. Cancer Res. 2004; 64:8736–8745. PMID: 15574785.
Article13. Dittmer D, Pati S, Zambetti G, Chu S, Teresky AK, Moore M, Finlay C, Levine AJ. Gain of function mutations in p53. Nat Genet. 1993; 4:42–46. PMID: 8099841.
Article14. da Silva SD, Cunha IW, Nishimoto IN, Soares FA, Carraro DM, Kowalski LP, Graner E. Clinicopathological significance of ubiquitin-specific protease 2a (USP2a), fatty acid synthase (FASN), and ErbB2 expression in oral squamous cell carcinomas. Oral Oncol. 2009; 45:e134–e139. PMID: 19362044.15. Graner E, Tang D, Rossi S, Baron A, Migita T, Weinstein LJ, Lechpammer M, Huesken D, Zimmermann J, Signoretti S, Loda M. The isopeptidase USP2a regulates the stability of fatty acid synthase in prostate cancer. Cancer Cell. 2004; 5:253–261. PMID: 15050917.
Article16. Sacco JJ, Coulson JM, Clague MJ, Urbé S. Emerging roles of deubiquitinases in cancer-associated pathways. IUBMB Life. 2010; 62:140–157. PMID: 20073038.
Article17. Nepal S, Shrestha A, Park PH. Ubiquitin specific protease 2 acts as a key modulator for the regulation of cell cycle by adiponectin and leptin in cancer cells. Mol Cell Endocrinol. 2015; 412:44–55. PMID: 26033248.
Article18. Nepal S, Kim MJ, Subedi A, Lee ES, Yong CS, Kim JA, Kang W, Kwak MK, Arya DS, Park PH. Globular adiponectin inhibits ethanol-induced apoptosis in HepG2 cells through heme oxygenase-1 induction. Biochem Pharmacol. 2012; 84:974–983. PMID: 22842631.
Article19. Nepal S, Park PH. Regulatory role of autophagy in globular adiponectin-induced apoptosis in cancer cells. Biomol Ther (Seoul). 2014; 22:384–389. PMID: 25414767.
Article20. Nepal S, Park PH. Activation of autophagy by globular adiponectin attenuates ethanol-induced apoptosis in HepG2 cells: involvement of AMPK/FoxO3A axis. Biochim Biophys Acta. 2013; 1833:2111–2125. PMID: 23688633.
Article21. Kim MJ, Nagy LE, Park PH. Globular adiponectin inhibits ethanolinduced reactive oxygen species production through modulation of NADPH oxidase in macrophages: involvement of liver kinase B1/AMP-activated protein kinase pathway. Mol Pharmacol. 2014; 86:284–296. PMID: 24850909.
Article22. Lee JT, Gu W. The multiple levels of regulation by p53 ubiquitination. Cell Death Differ. 2010; 17:86–92. PMID: 19543236.
Article23. Eskelinen EL. The dual role of autophagy in cancer. Curr Opin Pharmacol. 2011; 11:294–300. PMID: 21498118.
Article24. van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev. 2009; 18:2569–2578. PMID: 19755644.
Article25. Surmacz E. Obesity hormone leptin: a new target in breast cancer? Breast Cancer Res. 2007; 9:301. PMID: 17274833.
Article26. Kelesidis T, Kelesidis I, Chou S, Mantzoros CS. Narrative review: the role of leptin in human physiology: emerging clinical applications. Ann Intern Med. 2010; 152:93–100. PMID: 20083828.
Article27. Ptak A, Kolaczkowska E, Gregoraszczuk EL. Leptin stimulation of cell cycle and inhibition of apoptosis gene and protein expression in OVCAR-3 ovarian cancer cells. Endocrine. 2013; 43:394–403. PMID: 22968658.
Article28. DeLeo AB, Jay G, Appella E, Dubois GC, Law LW, Old LJ. Detection of a transformation-related antigen in chemically induced sarcomas and other transformed cells of the mouse. Proc Natl Acad Sci U S A. 1979; 76:2420–2424. PMID: 221923.
Article29. Jänicke RU, Sohn D, Schulze-Osthoff K. The dark side of a tumor suppressor: anti-apoptotic p53. Cell Death Differ. 2008; 15:959–976. PMID: 18356920.
Article30. Lin YC, Wang FF. Mechanisms underlying the pro-survival pathway of p53 in suppressing mitotic death induced by adriamycin. Cell Signal. 2008; 20:258–267. PMID: 18006273.
Article31. Oren M. Decision making by p53: life, death and cancer. Cell Death Differ. 2003; 10:431–442. PMID: 12719720.
Article32. Kastan MB. Wild-type p53: tumors can't stand it. Cell. 2007; 128:837–840. PMID: 17350571.
Article33. Sablina AA, Budanov AV, Ilyinskaya GV, Agapova LS, Kravchenko JE, Chumakov PM. The antioxidant function of the p53 tumor suppressor. Nat Med. 2005; 11:1306–1313. PMID: 16286925.
Article34. Lassus P, Ferlin M, Piette J, Hibner U. Anti-apoptotic activity of low levels of wild-type p53. EMBO J. 1996; 15:4566–4573. PMID: 8887548.
Article35. Oren M, Rotter V. Mutant p53 gain-of-function in cancer. Cold Spring Harb Perspect Biol. 2010; 2:a001107. PMID: 20182618.
Article36. Elledge RM, Lock-Lim S, Allred DC, Hilsenbeck SG, Cordner L. p53 mutation and tamoxifen resistance in breast cancer. Clin Cancer Res. 1995; 1:1203–1208. PMID: 9815913.37. Lim LY, Vidnovic N, Ellisen LW, Leong CO. Mutant p53 mediates survival of breast cancer cells. Br J Cancer. 2009; 101:1606–1612. PMID: 19773755.
Article38. Brito AF, Abrantes AM, Pinto-Costa C, Gomes AR, Mamede AC, Casalta-Lopes J, Gonçalves AC, Sarmento-Ribeiro AB, Tralhão JG, Botelho MF. Hepatocellular carcinoma and chemotherapy: the role of p53. Chemotherapy. 2012; 58:381–386. PMID: 23257706.
Article39. Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997; 88:323–331. PMID: 9039259.
Article40. O'Connor PM, Jackman J, Bae I, Myers TG, Fan S, Mutoh M, Scudiero DA, Monks A, Sausville EA, Weinstein JN, Friend S, Fornace AJ Jr, Kohn KW. Characterization of the p53 tumor suppressor pathway in cell lines of the National Cancer Institute anticancer drug screen and correlations with the growth-inhibitory potency of 123 anticancer agents. Cancer Res. 1997; 57:4285–4300. PMID: 9331090.41. Kastan MB, Onyekwere O, Sidransky D, Vogelstein B, Craig RW. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991; 51:6304–6311. PMID: 1933891.42. Nagasawa H, Li CY, Maki CG, Imrich AC, Little JB. Relationship between radiation-induced G1 phase arrest and p53 function in human tumor cells. Cancer Res. 1995; 55:1842–1846. PMID: 7728750.43. Nagasawa H, Keng P, Maki C, Yu Y, Little JB. Absence of a radiation-induced first-cycle G1-S arrest in p53+ human tumor cells synchronized by mitotic selection. Cancer Res. 1998; 58:2036–2041. PMID: 9581850.44. Little JB, Nagasawa H, Keng PC, Yu Y, Li CY. Absence of radiationinduced G1 arrest in two closely related human lymphoblast cell lines that differ in p53 status. J Biol Chem. 1995; 270:11033–11036. PMID: 7744731.
Article45. Li CY, Nagasawa H, Dahlberg WK, Little JB. Diminished capacity for p53 in mediating a radiation-induced G1 arrest in established human tumor cell lines. Oncogene. 1995; 11:1885–1892. PMID: 7478618.46. Park HJ, Lyons JC, Ohtsubo T, Song CW. Cell cycle progression and apoptosis after irradiation in an acidic environment. Cell Death Differ. 2000; 7:729–738. PMID: 10918447.
Article47. Bhutia SK, Mukhopadhyay S, Sinha N, Das DN, Panda PK, Patra SK, Maiti TK, Mandal M, Dent P, Wang XY, Das SK, Sarkar D, Fisher PB. Autophagy: cancer's friend or foe? Adv Cancer Res. 2013; 118:61–95. PMID: 23768510.48. Jain K, Paranandi KS, Sridharan S, Basu A. Autophagy in breast cancer and its implications for therapy. Am J Cancer Res. 2013; 3:251–265. PMID: 23841025.49. Samuel T, Weber HO, Rauch P, Verdoodt B, Eppel JT, McShea A, Hermeking H, Funk JO. The G2/M regulator 14-3-3sigma prevents apoptosis through sequestration of Bax. J Biol Chem. 2001; 276:45201–45206. PMID: 11574543.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diallyl Trisulfide Inhibits Leptin-induced OncogenicSignaling in Human Breast Cancer Cells but Fails toPrevent Chemically-induced Luminal-type Cancer in Rats
- Leptin and Leptin Receptor Expression in Breast Cancer
- Leptin as a Potential Target for Estrogen Receptor-Positive Breast Cancer
- Signaling Role of Adipocyte Leptin in Prostate Cell Proliferation Induced by Trichomonas vaginalis
- Leptin-signal transduction pathways and relationship with cancer development