J Vet Sci.
2015 Sep;16(3):281-287. 10.4142/jvs.2015.16.3.281.
Decreased expression of Toll-like receptor 4 and 5 during progression of prostate transformation in transgenic adenocarcinoma of mouse prostate mice
- Affiliations
-
- 1Laboratory Animal Medicine, BK21 PLUS Program for Creative Veterinary Science Research, Research Institute for Veterinary Science and College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea. pjhak@snu.ac.kr
- 2World Class Institute, Korea Research Institute of Bioscience and Biotechnology, Ochang 363-883, Korea.
- 3Department of Biochemistry, College of Medicine, Konyang University, Daejeon 302-718, Korea. kimdj77@gmail.com
- KMID: 2344300
- DOI: http://doi.org/10.4142/jvs.2015.16.3.281
Abstract
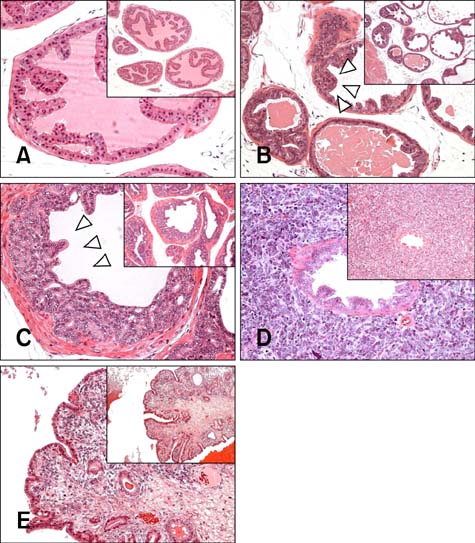
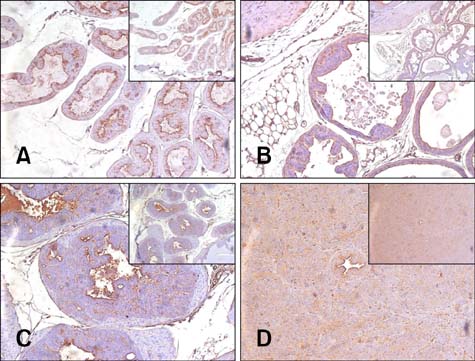
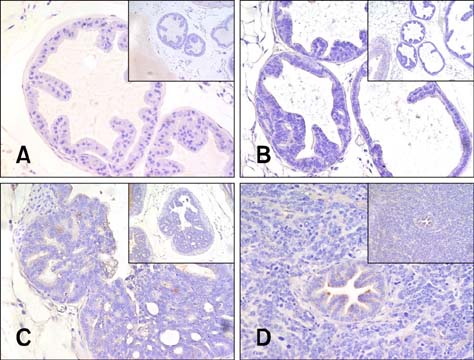
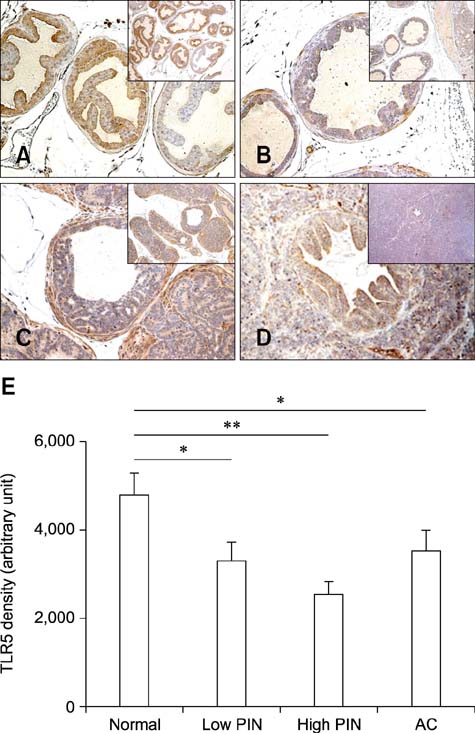
- Chronic inflammation has been considered an important risk factor for development of prostate cancer. Toll-like receptors (TLRs) recognize microbial moieties or endogenous molecules and play an important role in the triggering and promotion of inflammation. In this study, we examined whether expression of TLR4 and TLR5 was associated with progression of prostate transformation in the transgenic adenocarcinoma of mouse prostate (TRAMP) model. The expression of TLR4 and TLR5 was evaluated by immunohistochemisty in formalin-fixed paraffin-embedded prostate tissue from wild-type (WT) and TRAMP mice. Normal prostate tissue from WT mice showed strong expression of TLR4 and TLR5. However, TLR4 expression in the prostate tissue from TRAMP mice gradually decreased as pathologic grade became more aggressive. TLR5 expression in the prostate tissue from TRAMP mice also decreased in low-grade prostate intraepithelial neoplasia (PIN), high-grade PIN and poorly differentiated adenocarcinoma. Overall, our results suggest that decreased expression of TLR4 and TLR5 may contribute to prostate tumorigenesis.
Keyword
MeSH Terms
-
Adenocarcinoma/etiology/*genetics
Animals
Cell Transformation, Neoplastic
Disease Progression
*Gene Expression Regulation, Neoplastic
Humans
Male
Mice
Mice, Inbred C57BL
Mice, Transgenic
Prostatic Neoplasms/etiology/*genetics
Toll-Like Receptor 4/*genetics/metabolism
Toll-Like Receptor 5/*genetics/metabolism
Toll-Like Receptor 4
Toll-Like Receptor 5
Figure
Reference
-
1. Andersen-Nissen E, Hawn TR, Smith KD, Nachman A, Lampano AE, Uematsu S, Akira S, Aderem A. Cutting edge: Tlr5-/- mice are more susceptible to Escherichia coli urinary tract infection. J Immunol. 2007; 178:4717–4720.
Article2. Andreani V, Gatti G, Simonella L, Rivero V, Maccioni M. Activation of Toll-like receptor 4 on tumor cells in vitro inhibits subsequent tumor growth in vivo. Cancer Res. 2007; 67:10519–10527.
Article3. Cai T, Mazzoli S, Meacci F, Boddi V, Mondaini N, Malossini G, Bartoletti R. Epidemiological features and resistance pattern in uropathogens isolated from chronic bacterial prostatitis. J Microbiol. 2011; 49:448–454.
Article4. Cai Z, Sanchez A, Shi Z, Zhang T, Liu M, Zhang D. Activation of Toll-like receptor 5 on breast cancer cells by flagellin suppresses cell proliferation and tumor growth. Cancer Res. 2011; 71:2466–2475.
Article5. Chiaverotti T, Couto SS, Donjacour A, Mao JH, Nagase H, Cardiff RD, Cunha GR, Balmain A. Dissociation of epithelial and neuroendocrine carcinoma lineages in the transgenic adenocarcinoma of mouse prostate model of prostate cancer. Am J Pathol. 2008; 172:236–246.
Article6. De Marzo AM, Platz EA, Sutcliffe S, Xu J, Grönberg H, Drake CG, Nakai Y, Isaacs WB, Nelson WG. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007; 7:256–269.
Article7. El-Omar EM, Ng MT, Hold GL. Polymorphisms in Toll-like receptor genes and risk of cancer. Oncogene. 2008; 27:244–252.
Article8. Foster BA, Gingrich JR, Kwon ED, Madias C, Greenberg NM. Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res. 1997; 57:3325–3330.9. Galli R, Starace D, Busà R, Angelini DF, Paone A, De Cesaris P, Filippini A, Sette C, Battistini L, Ziparo E, Riccioli A. TLR stimulation of prostate tumor cells induces chemokine-mediated recruitment of specific immune cell types. J Immunol. 2010; 184:6658–6669.
Article10. Gatti G, Quintar AA, Andreani V, Nicola JP, Maldonado CA, Masini-Repiso AM, Rivero VE, Maccioni M. Expression of Toll-like receptor 4 in the prostate gland and its association with the severity of prostate cancer. Prostate. 2009; 69:1387–1397.
Article11. Gatti G, Rivero V, Motrich RD, Maccioni M. Prostate epithelial cells can act as early sensors of infection by upregulating TLR4 expression and proinflammatory mediators upon LPS stimulation. J Leukoc Biol. 2006; 79:989–998.
Article12. González-Reyes S, Fernández JM, González LO, Aguirre A, Suárez A, González JM, Escaff S, Vizoso FJ. Study of TLR3, TLR4, and TLR9 in prostate carcinomas and their association with biochemical recurrence. Cancer Immunol Immunother. 2011; 60:217–226.
Article13. González-Reyes S, Marín L, González L, González LO, del Casar JM, Lamelas ML, González-Quintana JM, Vizoso FJ. Study of TLR3, TLR4 and TLR9 in breast carcinomas and their association with metastasis. BMC Cancer. 2010; 10:665.
Article14. Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ, Rosen JM. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci U S A. 1995; 92:3439–3443.
Article15. Han JH, Park SY, Kim JB, Cho SD, Kim B, Kim BY, Kang MJ, Kim DJ, Park JH, Park JH. TLR7 expression is decreased during tumour progression in transgenic adenocarcinoma of mouse prostate mice and its activation inhibits growth of prostate cancer cells. Am J Reprod Immunol. 2013; 70:317–326.
Article16. He W, Liu Q, Wang L, Chen W, Li N, Cao X. TLR4 signaling promotes immune escape of human lung cancer cells by inducing immunosuppressive cytokines and apoptosis resistance. Mol Immunol. 2007; 44:2850–2859.
Article17. Ittmann M, Huang J, Radaelli E, Martin P, Signoretti S, Sullivan R, Simons BW, Ward JM, Robinson BD, Chu GC, Loda M, Thomas G, Borowsky A, Cardiff RD. Animal models of human prostate cancer: the consensus report of the New York meeting of the mouse models of human cancers consortium prostate pathology committee. Cancer Res. 2013; 73:2718–2736.
Article18. Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004; 5:987–995.
Article19. König JE, Senge T, Allhoff EP, König W. Analysis of the inflammatory network in benign prostate hyperplasia and prostate cancer. Prostate. 2004; 58:121–129.
Article20. Kaplan-Lefko PJ, Chen TM, Ittmann MM, Barrios RJ, Ayala GE, Huss WJ, Maddison LA, Foster BA, Greenberg NM. Pathobiology of autochthonous prostate cancer in a preclinical transgenic mouse model. Prostate. 2003; 55:219–237.
Article21. Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011; 34:637–650.
Article22. Kim WY, Lee JW, Choi JJ, Choi CH, Kim TJ, Kim BG, Song SY, Bae DS. Increased expression of Toll-like receptor 5 during progression of cervical neoplasia. Int J Gynecol Cancer. 2008; 18:300–305.
Article23. Matijevic T, Marjanovic M, Pavelic J. Functionally active Toll-like receptor 3 on human primary and metastatic cancer cells. Scand J Immunol. 2009; 70:18–24.
Article24. Nomi N, Kodama S, Suzuki M. Toll-like receptor 3 signaling induces apoptosis in human head and neck cancer via survivin associated pathway. Oncol Rep. 2010; 24:225–231.
Article25. Omabe M, Ezeani M. Infection, inflammation and prostate carcinogenesis. Infect Genet Evol. 2011; 11:1195–1198.
Article26. Park JH, Walls JE, Galvez JJ, Kim M, Abate-Shen C, Shen MM, Cardiff RD. Prostatic intraepithelial neoplasia in genetically engineered mice. Am J Pathol. 2002; 161:727–735.
Article27. Park JH, Yoon HE, Kim DJ, Kim SA, Ahn SG, Yoon JH. Toll-like receptor 5 activation promotes migration and invasion of salivary gland adenocarcinoma. J Oral Pathol Med. 2011; 40:187–193.
Article28. Pei Z, Lin D, Song X, Li H, Yao H. TLR4 signaling promotes the expression of VEGF and TGFβ1 in human prostate epithelial PC3 cells induced by lipopolysaccharide. Cell Immunol. 2008; 254:20–27.
Article29. Persson BE, Ronquist G. Evidence for a mechanistic association between nonbacterial prostatitis and levels of urate and creatinine in expressed prostatic secretion. J Urol. 1996; 155:958–960.
Article30. Rhee SH, Im E, Pothoulakis C. Toll-like receptor 5 engagement modulates tumor development and growth in a mouse xenograft model of human colon cancer. Gastroenterology. 2008; 135:518–528.
Article31. Riddell JR, Bshara W, Moser MT, Spernyak JA, Foster BA, Gollnick SO. Peroxiredoxin 1 controls prostate cancer growth through Toll-like receptor 4-dependent regulation of tumor vasculature. Cancer Res. 2011; 71:1637–1646.
Article32. Sarma AV, McLaughlin JC, Wallner LP, Dunn RL, Cooney KA, Schottenfeld D, Montie JE, Wei JT. Sexual behavior, sexually transmitted diseases and prostatitis: the risk of prostate cancer in black men. J Urol. 2006; 176:1108–1113.
Article33. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012; 62:10–29.
Article34. Song EJ, Kang MJ, Kim YS, Kim SM, Lee SE, Kim CH, Kim DJ, Park JH. Flagellin promotes the proliferation of gastric cancer cells via the Toll-like receptor 5. Int J Mol Med. 2011; 28:115–119.
Article35. Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003; 21:335–376.
Article36. Takeyama K, Mitsuzawa H, Shimizu T, Konishi M, Nishitani C, Sano H, Kunishima Y, Matsukawa M, Takahashi S, Shibata K, Tsukamoto T, Kuroki Y. Prostate cell lines secrete IL-8 in response to Mycoplasma hominis through Toll-like receptor 2-mediated mechanism. Prostate. 2006; 66:386–391.
Article37. Väisänen MR, Väisänen T, Jukkola-Vuorinen A, Vuopala KS, Desmond R, Selander KS, Vaarala MH. Expression of Toll-like receptor-9 is increased in poorly differentiated prostate tumors. Prostate. 2010; 70:817–824.
Article38. Wang H, Rayburn ER, Wang W, Kandimalla ER, Agrawal S, Zhang R. Chemotherapy and chemosensitization of nonsmall cell lung cancer with a novel immunomodulatory oligonucleotide targeting Toll-like receptor 9. Mol Cancer Ther. 2006; 5:1585–1592.
Article39. Wang L, Zhu R, Huang Z, Li H, Zhu H. Lipopolysaccharide-induced Toll-like receptor 4 signaling in cancer cells promotes cell survival and proliferation in hepatocellular carcinoma. Dig Dis Sci. 2013; 58:2223–2236.
Article40. Yuan TC, Veeramani S, Lin MF. Neuroendocrine-like prostate cancer cells: neuroendocrine transdifferentiation of prostate adenocarcinoma cells. Endocr Relat Cancer. 2007; 14:531–547.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of dietary high fat on prostate intraepithelial neoplasia in TRAMP mice
- Ductal Adenocarcinoma of Prostate
- High-Grade Prostatic Intraepithelial Neoplasia
- Prevention of Prostate Cancer in Transgenic Adenocarcinoma of the Mouse Prostate Mice by Yellow Passion Fruit Extract and Antiproliferative Effects of Its Bioactive Compound Piceatannol
- Change of serum prostate specific antigen values after radiation therapy in prostate cancer