Lab Anim Res.
2013 Mar;29(1):39-47. 10.5625/lar.2013.29.1.39.
Effects of dietary high fat on prostate intraepithelial neoplasia in TRAMP mice
- Affiliations
-
- 1Department of Laboratory Animal Medicine, College of Veterinary Medicine, Seoul National University, Seoul, Korea. pjhak@snu.ac.kr
- 2Biological Resources Coordination Division, National Institute of Biological Resources, Incheon, Korea.
- 3Department of Applied Bioscience, CHA University, Seoul, Korea.
- KMID: 2312102
- DOI: http://doi.org/10.5625/lar.2013.29.1.39
Abstract
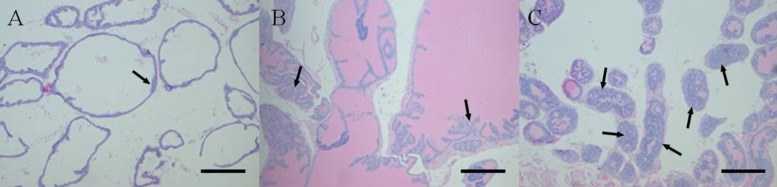
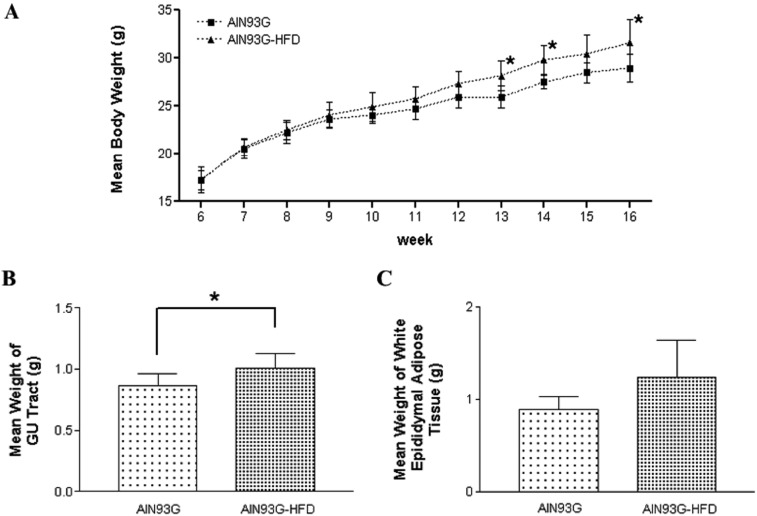
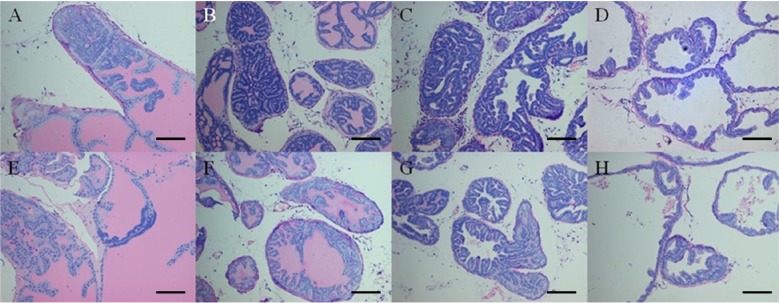
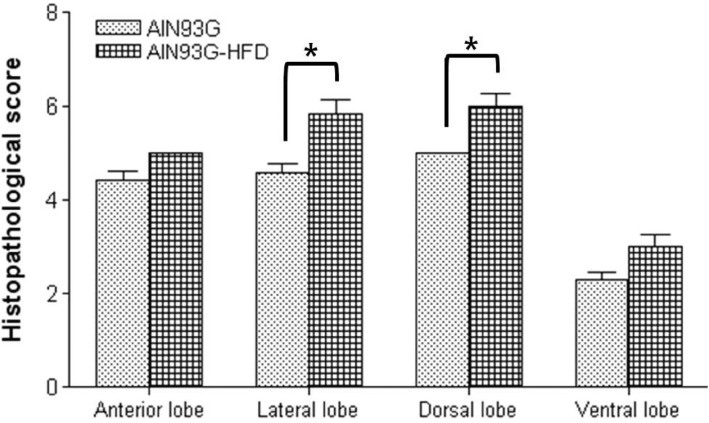
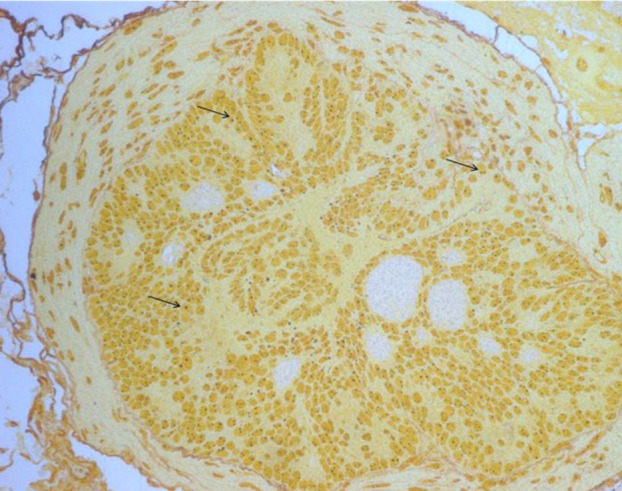
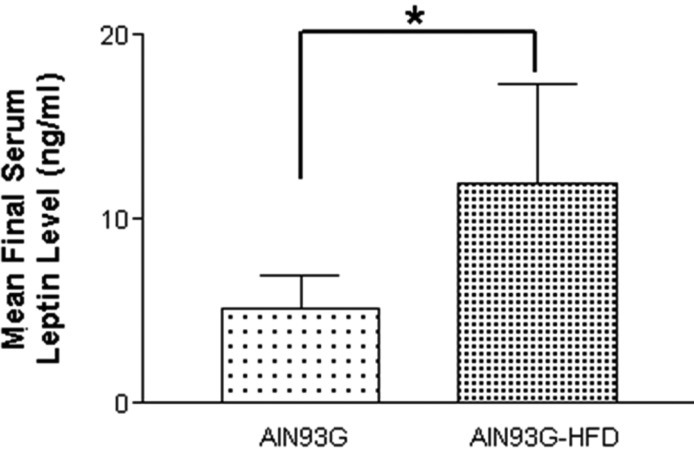
- Increased fat intake is known to be a major cause of prostate cancer. In this study, we investigated the effect of dietary high fat on prostate intraepithelial neoplasia using transgenic adenocarcinoma mouse prostate (TRAMP) mice. Six-week-old male TRAMP mice were fed AIN93G (control group, 4.0 kcal/kg, n=6) and AIN93G-HFD (experimental group, 4.8 kcal/kg, n=7) for 10 weeks. Prostate histopathology, urogenital tract (UGT) weight, epididymal white adipose tissue weight, argyrophilic nucleolar organizer regions (AgNORs) counts, and serum leptin levels were examined. AIN93G-HFD fed group showed progressed neoplastic lesions in the prostate (P<0.05) compared to AIN93G fed group. AIN93G-HFD intake resulted in a increase in the weight of UGT (P<0.05) and epididymal white adipose tissue. The number of Ag-NOR positive dots significantly increased in each prostate lobe and final serum leptin levels in AIN93G-HFD fed group were about twice those of AIN93G fed group (P<0.05). Dietary high fat was related to the prostate cancer progression in the early stage of TRAMP mice and increased serum leptin levels, suggesting that the regulation of dietary components could delay the progression of prostate cancer.
Keyword
MeSH Terms
Figure
Reference
-
1. Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007; 57(1):43–66. PMID: 17237035.
Article2. Sim HG, Cheng CW. Changing demography of prostate cancer in Asia. Eur J Cancer. 2005; 41(6):834–845. PMID: 15808953.
Article3. Pu YS, Chiang HS, Lin CC, Huang CY, Huang KH, Chen J. Changing trends of prostate cancer in Asia. Aging Male. 2004; 7(2):120–132. PMID: 15672937.
Article4. Bravo MP, Castellanos E, del Rey Calero J. Dietary factors and prostatic cancer. Urol Int. 1991; 46(2):163–166. PMID: 2053225.
Article5. Gann PH, Hennekens CH, Sacks FM, Grodstein F, Giovannucci EL, Stampfer MJ. Prospective study of plasma fatty acids and risk of prostate cancer. J Natl Cancer Inst. 1994; 86(4):281–286. PMID: 8158682.
Article6. Graham S, Haughey B, Marshall J, Priore R, Byers T, Rzepka T, Mettlin C, Pontes JE. Diet in the epidemiology of carcinoma of the prostate gland. J Natl Cancer Inst. 1983; 70(4):687–692. PMID: 6572757.7. Whittemore AS, Kolonel LN, Wu AH, John EM, Gallagher RP, Howe GR, Burch JD, Hankin J, Dreon DM, West DW, et al. Prostate cancer in relation to diet, physical activity, and body size in blacks, whites, and Asians in the United States and Canada. J Natl Cancer Inst. 1995; 87(9):652–661. PMID: 7752270.
Article8. Pollard M, Luckert PH. Promotional effects of testosterone and dietary fat on prostate carcinogenesis in genetically susceptible rats. Prostate. 1985; 6(1):1–5. PMID: 3969369.9. Pollard M, Luckert PH. Promotional effects of testosterone and high fat diet on the development of autochthonous prostate cancer in rats. Cancer Lett. 1986; 32(2):223–227. PMID: 3756849.
Article10. Tamano S, Rehm S, Waalkes MP, Ward JM. High incidence and histogenesis of seminal vesicle adenocarcinoma and lower incidence of prostate carcinomas in the Lobund-Wistar prostate cancer rat model using N-nitrosomethylurea and testosterone. Vet Pathol. 1996; 33(5):557–567. PMID: 8885183.
Article11. Wang Y, Corr JG, Thaler HT, Tao Y, Fair WR, Heston WD. Decreased growth of established human prostate LNCaP tumors in nude mice fed a low-fat diet. J Natl Cancer Inst. 1995; 87(19):1456–1462. PMID: 7545759.
Article12. Carroll KK, Noble RL. Dietary fat in relation to hormonal induction of mammary and prostatic carcinoma in Nb rats. Carcinogenesis. 1987; 8(6):851–853. PMID: 3608084.
Article13. Pour PM, Groot K, Kazakoff K, Anderson K, Schally AV. Effects of high-fat diet on the patterns of prostatic cancer induced in rats by N-nitrosobis(2-oxopropyl)amine and testosterone. Cancer Res. 1991; 51(18):4757–4761. PMID: 1909929.14. Shirai T, Tamano S, Kato T, Iwasaki S, Takahashi S, Ito N. Induction of invasive carcinomas in the accessory sex organs other than the ventral prostate of rats given 3,2'-dimethyl-4-aminobiphenyl and testosterone propionate. Cancer Res. 1991; 51(4):1264–1269. PMID: 1997167.15. Bodmer WF. Prostate cancer 2000. Prostate Cancer Prostatic Dis. 2000; 3(4):218–223. PMID: 12497067.
Article16. Gingrich JR, Greenberg NM. A transgenic mouse prostate cancer model. Toxicol Pathol. 1996; 24(4):502–504. PMID: 8864193.
Article17. Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ, Rosen JM. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci U S A. 1995; 92(8):3439–3443. PMID: 7724580.
Article18. Gingrich JR, Barrios RJ, Morton RA, Boyce BF, DeMayo FJ, Finegold MJ, Angelopoulou R, Rosen JM, Greenberg NM. Metastatic prostate cancer in a transgenic mouse. Cancer Res. 1996; 56(18):4096–4102. PMID: 8797572.19. Park JH, Walls JE, Galvez JJ, Kim M, Abate-Shen C, Shen MM, Cardiff RD. Prostatic intraepithelial neoplasia in genetically engineered mice. Am J Pathol. 2002; 161(2):727–735. PMID: 12163397.
Article20. Ploton D, Menager M, Jeannesson P, Himber G, Pigeon F, Adnet JJ. Improvement in the staining and in the visualization of the argyrophilic proteins of the nucleolar organizer region at the optical level. Histochem J. 1986; 18(1):5–14. PMID: 2423479.
Article21. Bray GA. The underlying basis for obesity: relationship to cancer. J Nutr. 2002; 132(11 Suppl):3451S–3455S. PMID: 12421869.
Article22. Kobayashi K. Adipokines: therapeutic targets for metabolic syndrome. Curr Drug Targets. 2005; 6(4):525–529. PMID: 16026271.
Article23. Kennedy A, Gettys TW, Watson P, Wallace P, Ganaway E, Pan Q, Garvey WT. The metabolic significance of leptin in humans: gender-based differences in relationship to adiposity, insulin sensitivity, and energy expenditure. J Clin Endocrinol Metab. 1997; 82(4):1293–1300. PMID: 9100610.
Article24. Dieudonne MN, Machinal-Quelin F, Serazin-Leroy V, Leneveu MC, Pecquery R, Giudicelli Y. Leptin mediates a proliferative response in human MCF7 breast cancer cells. Biochem Biophys Res Commun. 2002; 293(1):622–628. PMID: 12054648.
Article25. Hu X, Juneja SC, Maihle NJ, Cleary MP. Leptin--a growth factor in normal and malignant breast cells and for normal mammary gland development. J Natl Cancer Inst. 2002; 94(22):1704–1711. PMID: 12441326.
Article26. Attoub S, Noe V, Pirola L, Bruyneel E, Chastre E, Mareel M, Wymann MP, Gespach C. Leptin promotes invasiveness of kidney and colonic epithelial cells via phosphoinositide 3-kinase-, rho-, and rac-dependent signaling pathways. FASEB J. 2000; 14(14):2329–2338. PMID: 11053255.
Article27. Hardwick JC, Van Den Brink GR, Offerhaus GJ, Van Deventer SJ, Peppelenbosch MP. Leptin is a growth factor for colonic epithelial cells. Gastroenterology. 2001; 121(1):79–90. PMID: 11438496.
Article28. Briscoe CP, Hanif S, Arch JR, Tadayyon M. Fatty acids inhibit leptin signalling in BRIN-BD11 insulinoma cells. J Mol Endocrinol. 2001; 26(2):145–154. PMID: 11241166.
Article29. Okuya S, Tanabe K, Tanizawa Y, Oka Y. Leptin increases the viability of isolated rat pancreatic islets by suppressing apoptosis. Endocrinology. 2001; 142(11):4827–4830. PMID: 11606450.
Article30. Choi JH, Park SH, Leung PC, Choi KC. Expression of leptin receptors and potential effects of leptin on the cell growth and activation of mitogen-activated protein kinases in ovarian cancer cells. J Clin Endocrinol Metab. 2005; 90(1):207–210. PMID: 15522945.
Article31. Tsuchiya T, Shimizu H, Horie T, Mori M. Expression of leptin receptor in lung: leptin as a growth factor. Eur J Pharmacol. 1999; 365(2-3):273–279. PMID: 9988112.
Article32. Han C, Zhang HT, Du L, Liu X, Jing J, Zhao X, Yang X, Tian B. Serum levels of leptin, insulin, and lipids in relation to breast cancer in china. Endocrine. 2005; 26(1):19–24. PMID: 15805581.
Article33. Tessitore L, Vizio B, Jenkins O, De Stefano I, Ritossa C, Argiles JM, Benedetto C, Mussa A. Leptin expression in colorectal and breast cancer patients. Int J Mol Med. 2000; 5(4):421–426. PMID: 10719061.
Article34. Stattin P, Palmqvist R, Söderberg S, Biessy C, Ardnor B, Hallmans G, Kaaks R, Olsson T. Plasma leptin and colorectal cancer risk: a prospective study in Northern Sweden. Oncol Rep. 2003; 10(6):2015–2021. PMID: 14534736.
Article35. Petridou E, Belechri M, Dessypris N, Koukoulomatis P, Diakomanolis E, Spanos E, Trichopoulos D. Leptin and body mass index in relation to endometrial cancer risk. Ann Nutr Metab. 2002; 46(3-4):147–151. PMID: 12169858.
Article36. Yuan SS, Tsai KB, Chung YF, Chan TF, Yeh YT, Tsai LY, Su JH. Aberrant expression and possible involvement of the leptin receptor in endometrial cancer. Gynecol Oncol. 2004; 92(3):769–775. PMID: 14984939.
Article37. Somasundar P, Yu AK, Vona-Davis L, McFadden DW. Differential effects of leptin on cancer in vitro. J Surg Res. 2003; 113(1):50–55. PMID: 12943810.
Article38. Somasundar P, Frankenberry KA, Skinner H, Vedula G, McFadden DW, Riggs D, Jackson B, Vangilder R, Hileman SM, Vona-Davis LC. Prostate cancer cell proliferation is influenced by leptin. J Surg Res. 2004; 118(1):71–82. PMID: 15093720.40. Dong H, Bertler C, Schneider E, Ritter MA. Assessment of cell proliferation by AgNOR scores and Ki-67 labeling indices and a comparison with potential doubling times. Cytometry. 1997; 28(4):280–288. PMID: 9266747.
Article41. Miyazaki S, Sasano H, Suzuki T, Sawai T, Nishihira T, Mori S. Nucleolar organizer regions in human esophageal disorders: comparison with proliferating cell nuclear antigen by immunostaining. Mod Pathol. 1992; 5(4):396–401. PMID: 1353881.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- High-Grade Prostatic Intraepithelial Neoplasia
- Decreased expression of Toll-like receptor 4 and 5 during progression of prostate transformation in transgenic adenocarcinoma of mouse prostate mice
- Significance of Atypical Small Acinar Proliferation and High-Grade Prostatic Intraepithelial Neoplasia in Prostate Biopsy
- Expression of Prostatic Carcinoma Oncogene PTI - 1 in Prostatic Carcinoma , Prostatic Intraepithelial Neoplasia and Benign Prostatic Hyperplasia Using in situ PCR
- Clinical significance of prostatic intraepithelial neoplasia