J Korean Med Sci.
2015 Oct;30(10):1496-1502. 10.3346/jkms.2015.30.10.1496.
Effects of Repetitive Transcranial Magnetic Stimulation on Behavioral Recovery during Early Stage of Traumatic Brain Injury in Rats
- Affiliations
-
- 1Department of Physical Medicine & Rehabilitation, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 2Department of Neurology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Medical Research Institute, Regenerative & Neuroscience Laboratory, Kangbuk Samsung Hospital, Seoul, Korea.
- 4Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 5Department of Rehabilitation Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. mhchun@amc.seoul.kr
- KMID: 2344185
- DOI: http://doi.org/10.3346/jkms.2015.30.10.1496
Abstract
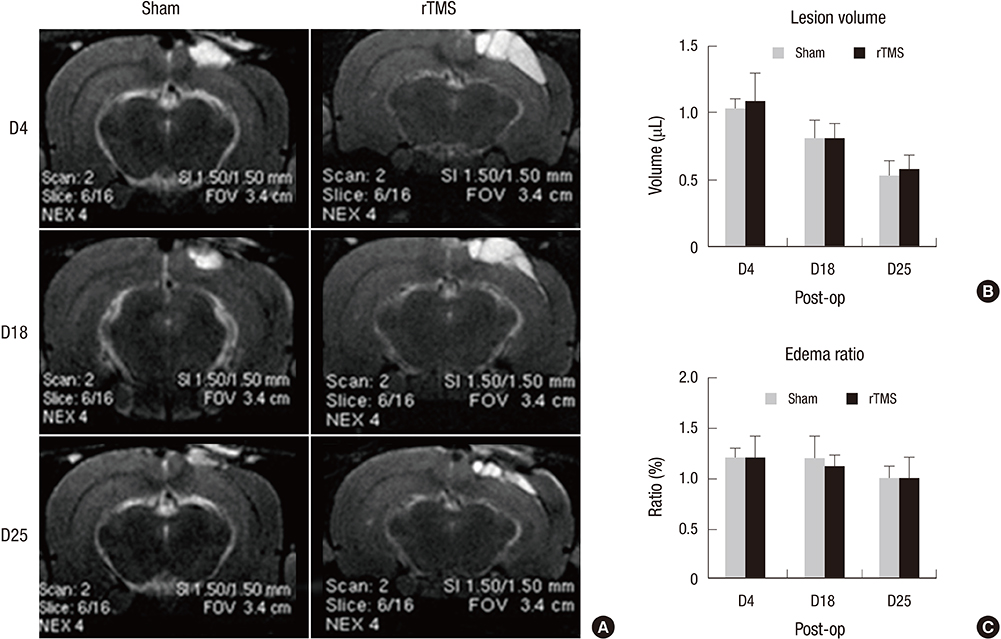
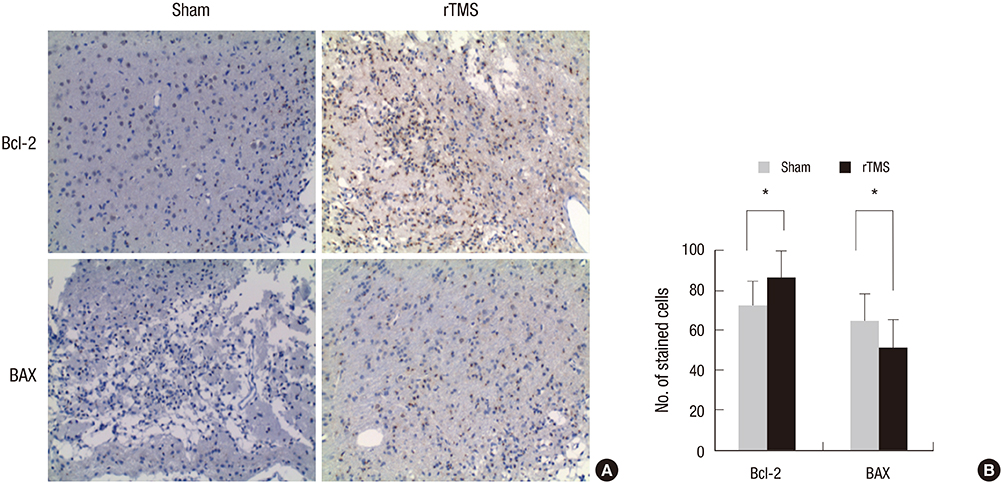
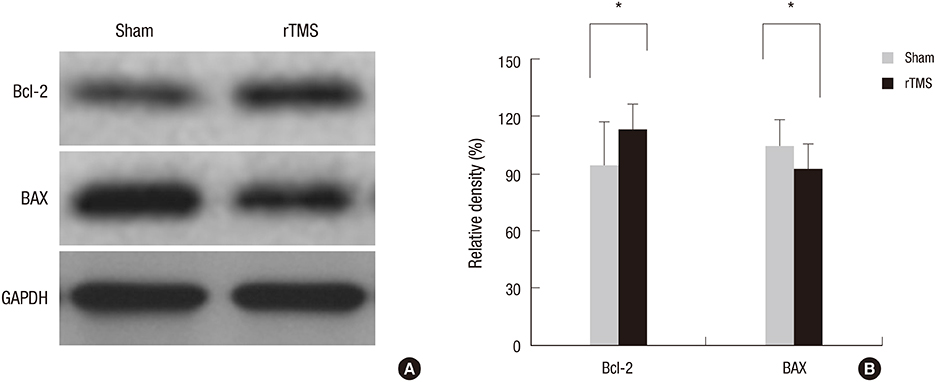
- Repetitive transcranial magnetic stimulation (rTMS) is a promising technique that modulates neural networks. However, there were few studies evaluating the effects of rTMS in traumatic brain injury (TBI). Herein, we assessed the effectiveness of rTMS on behavioral recovery and metabolic changes using brain magnetic resonance spectroscopy (MRS) in a rat model of TBI. We also evaluated the safety of rTMS by measuring brain swelling with brain magnetic resonance imaging (MRI). Twenty male Sprague-Dawley rats underwent lateral fluid percussion and were randomly assigned to the sham (n=10) or the rTMS (n=10) group. rTMS was applied on the fourth day after TBI and consisted of 10 daily sessions for 2 weeks with 10 Hz frequency (total pulses=3,000). Although the rTMS group showed an anti-apoptotic effect around the peri-lesional area, functional improvements were not significantly different between the two groups. Additionally, rTMS did not modulate brain metabolites in MRS, nor was there any change of brain lesion or edema after magnetic stimulation. These data suggest that rTMS did not have beneficial effects on motor recovery during early stages of TBI, although an anti-apoptosis was observed in the peri-lesional area.
Keyword
MeSH Terms
Figure
Reference
-
1. Ragnarsson KT. Results of the NIH consensus conference on "rehabilitation of persons with traumatic brain injury". Restor Neurol Neurosci. 2002; 20:103–108.2. Raghupathi R, Graham DI, McIntosh TK. Apoptosis after traumatic brain injury. J Neurotrauma. 2000; 17:927–938.3. Miñambres E, Ballesteros MA, Mayorga M, Marin MJ, Muñoz P, Figols J, López-Hoyos M. Cerebral apoptosis in severe traumatic brain injury patients: an in vitro, in vivo, and postmortem study. J Neurotrauma. 2008; 25:581–591.4. Knoblach SM, Alroy DA, Nikolaeva M, Cernak I, Stoica BA, Faden AI. Caspase inhibitor z-DEVD-fmk attenuates calpain and necrotic cell death in vitro and after traumatic brain injury. J Cereb Blood Flow Metab. 2004; 24:1119–1132.5. Soustiel JF, Palzur E, Nevo O, Thaler I, Vlodavsky E. Neuroprotective anti-apoptosis effect of estrogens in traumatic brain injury. J Neurotrauma. 2005; 22:345–352.6. Monnerie H, Tang-Schomer MD, Iwata A, Smith DH, Kim HA, Le Roux PD. Dendritic alterations after dynamic axonal stretch injury in vitro. Exp Neurol. 2010; 224:415–423.7. Hu B, Liu C, Bramlett H, Sick TJ, Alonso OF, Chen S, Dietrich WD. Changes in trkB-ERK1/2-CREB/Elk-1 pathways in hippocampal mossy fiber organization after traumatic brain injury. J Cereb Blood Flow Metab. 2004; 24:934–943.8. Chouinard PA, Van Der Werf YD, Leonard G, Paus T. Modulating neural networks with transcranial magnetic stimulation applied over the dorsal premotor and primary motor cortices. J Neurophysiol. 2003; 90:1071–1083.9. Siebner HR, Peller M, Willoch F, Minoshima S, Boecker H, Auer C, Drzezga A, Conrad B, Bartenstein P. Lasting cortical activation after repetitive TMS of the motor cortex: a glucose metabolic study. Neurology. 2000; 54:956–963.10. Strafella AP, Paus T. Cerebral blood-flow changes induced by paired-pulse transcranial magnetic stimulation of the primary motor cortex. J Neurophysiol. 2001; 85:2624–2629.11. Khedr EM, Ahmed MA, Fathy N, Rothwell JC. Therapeutic trial of repetitive transcranial magnetic stimulation after acute ischemic stroke. Neurology. 2005; 65:466–468.12. Mansur CG, Fregni F, Boggio PS, Riberto M, Gallucci-Neto J, Santos CM, Wagner T, Rigonatti SP, Marcolin MA, Pascual-Leone A. A sham stimulation-controlled trial of rTMS of the unaffected hemisphere in stroke patients. Neurology. 2005; 64:1802–1804.13. Takeuchi N, Chuma T, Matsuo Y, Watanabe I, Ikoma K. Repetitive transcranial magnetic stimulation of contralesional primary motor cortex improves hand function after stroke. Stroke. 2005; 36:2681–2686.14. Gao F, Wang S, Guo Y, Wang J, Lou M, Wu J, Ding M, Tian M, Zhang H. Protective effects of repetitive transcranial magnetic stimulation in a rat model of transient cerebral ischaemia: a microPET study. Eur J Nucl Med Mol Imaging. 2010; 37:954–961.15. Yoon KJ, Lee YT, Han TR. Mechanism of functional recovery after repetitive transcranial magnetic stimulation (rTMS) in the subacute cerebral ischemic rat model: neural plasticity or anti-apoptosis? Exp Brain Res. 2011; 214:549–556.16. McIntosh TK, Vink R, Noble L, Yamakami I, Fernyak S, Soares H, Faden AL. Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience. 1989; 28:233–244.17. Fonoff ET, Pereira JF Jr, Camargo LV, Dale CS, Pagano RL, Ballester G, Teixeira MJ. Functional mapping of the motor cortex of the rat using transdural electrical stimulation. Behav Brain Res. 2009; 202:138–141.18. Ogiue-Ikeda M, Kawato S, Ueno S. The effect of repetitive transcranial magnetic stimulation on long-term potentiation in rat hippocampus depends on stimulus intensity. Brain Res. 2003; 993:222–226.19. Fitzgerald PB, Brown TL, Daskalakis ZJ, Chen R, Kulkarni J. Intensity-dependent effects of 1 Hz rTMS on human corticospinal excitability. Clin Neurophysiol. 2002; 113:1136–1141.20. Kline RA, Negendank WG, McCoy LE, Lester M, Berguer R. MRI quantitation of edema in focal cerebral ischemia in cats: correlation with cytochrome aa3 oxidation state. Magn Reson Med. 1990; 13:319–323.21. Arnold DL, Matthews PM. Practical aspects of clinical applications of MRS in the brain. In : Charles CH, Young IR, editors. MR spectroscopy: clinical applications and techniques. London: Martin Dunitz;1996. p. 139–159.22. Antonsson B. Bax and other pro-apoptotic Bcl-2 family "killer-proteins" and their victim the mitochondrion. Cell Tissue Res. 2001; 306:347–361.23. Ginsberg MD, Zhao W, Alonso OF, Loor-Estades JY, Dietrich WD, Busto R. Uncoupling of local cerebral glucose metabolism and blood flow after acute fluid-percussion injury in rats. Am J Physiol. 1997; 272:H2859–H2868.24. Charriaut-Marlangue C, Margaill I, Represa A, Popovici T, Plotkine M, Ben-Ari Y. Apoptosis and necrosis after reversible focal ischemia: an in situ DNA fragmentation analysis. J Cereb Blood Flow Metab. 1996; 16:186–194.25. Clemens JA, Stephenson DT, Smalstig EB, Dixon EP, Little SP. Global ischemia activates nuclear factor-kappa B in forebrain neurons of rats. Stroke. 1997; 28:1073–1080. discussion 1080-126. Vexler ZS, Roberts TP, Bollen AW, Derugin N, Arieff AI. Transient cerebral ischemia. Association of apoptosis induction with hypoperfusion. J Clin Invest. 1997; 99:1453–1459.27. Clark RS, Chen J, Watkins SC, Kochanek PM, Chen M, Stetler RA, Loeffert JE, Graham SH. Apoptosis-suppressor gene bcl-2 expression after traumatic brain injury in rats. J Neurosci. 1997; 17:9172–9182.28. Reed JC. Proapoptotic multidomain Bcl-2/Bax-family proteins: mechanisms, physiological roles, and therapeutic opportunities. Cell Death Differ. 2006; 13:1378–1386.29. Verma A. Opportunities for neuroprotection in traumatic brain injury. J Head Trauma Rehabil. 2000; 15:1149–1161.30. Raghupathi R, Fernandez SC, Murai H, Trusko SP, Scott RW, Nishioka WK, McIntosh TK. BCL-2 overexpression attenuates cortical cell loss after traumatic brain injury in transgenic mice. J Cereb Blood Flow Metab. 1998; 18:1259–1269.31. Nakamura M, Raghupathi R, Merry DE, Scherbel U, Saatman KE, McIntosh TK. Overexpression of Bcl-2 is neuroprotective after experimental brain injury in transgenic mice. J Comp Neurol. 1999; 412:681–692.32. Tehranian R, Rose ME, Vagni V, Griffith RP, Wu S, Maits S, Zhang X, Clark RS, Dixon CE, Kochanek PM, et al. Transgenic mice that overexpress the anti-apoptotic Bcl-2 protein have improved histological outcome but unchanged behavioral outcome after traumatic brain injury. Brain Res. 2006; 1101:126–135.33. Humm JL, Kozlowski DA, Bland ST, James DC, Schallert T. Use-dependent exaggeration of brain injury: is glutamate involved? Exp Neurol. 1999; 157:349–358.34. Risedal A, Zeng J, Johansson BB. Early training may exacerbate brain damage after focal brain ischemia in the rat. J Cereb Blood Flow Metab. 1999; 19:997–1003.35. Katz DI, Alexander MP, Klein RB. Recovery of arm function in patients with paresis after traumatic brain injury. Arch Phys Med Rehabil. 1998; 79:488–493.36. Jang SH. Review of motor recovery in patients with traumatic brain injury. NeuroRehabilitation. 2009; 24:349–353.37. Kornienko VN, Pronin IN. Diagnostic neuroradiology. Berlin: Springer-Verlag;2009. p. 18–19.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Safety Review for Clinical Application of Repetitive Transcranial Magnetic Stimulation
- Effect of Epidural Electrical Stimulation and Repetitive Transcranial Magnetic Stimulation in Rats With Diffuse Traumatic Brain Injury
- Application of Non-invasive Brain Stimulation on Dysphagia after Stroke
- Effects of Electric Cortical Stimulation (ECS) and Transcranial Direct Current Stimulation (tDCS) on Rats With a Traumatic Brain Injury
- Noninvasive brain stimulation: repetitive transcranial magnetic stimulation and transcranial direct current stimulation