Ann Rehabil Med.
2015 Jun;39(3):416-424. 10.5535/arm.2015.39.3.416.
Effect of Epidural Electrical Stimulation and Repetitive Transcranial Magnetic Stimulation in Rats With Diffuse Traumatic Brain Injury
- Affiliations
-
- 1Department of Rehabilitation Medicine, Presbyterian Medical Center, Seonam University College of Medicine, Jeonju, Korea. lee@seonam.ac.kr
- 2Department of Medical Device Clinical Trial Center, Presbyterian Medical Center, Jeonju, Korea.
- 3Department of Rehabilitation Medicine, Chungnam National University School of Medicine, Daejeon, Korea.
- 4Department of Radiology, Presbyterian Medical Center, Seonam University College of Medicine, Jeonju, Korea.
- KMID: 2165645
- DOI: http://doi.org/10.5535/arm.2015.39.3.416
Abstract
OBJECTIVE
To evaluate the effects of epidural electrical stimulation (EES) and repetitive transcranial magnetic stimulation (rTMS) on motor recovery and brain activity in a rat model of diffuse traumatic brain injury (TBI) compared to the control group.
METHODS
Thirty rats weighing 270-285 g with diffuse TBI with 45 kg/cm2 using a weight-drop model were assigned to one of three groups: the EES group (ES) (anodal electrical stimulation at 50 Hz), the rTMS group (MS) (magnetic stimulation at 10 Hz, 3-second stimulation with 6-second intervals, 4,000 total stimulations per day), and the sham-treated control group (sham) (no stimulation). They were pre-trained to perform a single-pellet reaching task (SPRT) and a rotarod test (RRT) for 14 days. Diffuse TBI was then induced and an electrode was implanted over the dominant motor cortex. The changes in SPRT success rate, RRT performance time rate and the expression of c-Fos after two weeks of EES or rTMS were tracked.
RESULTS
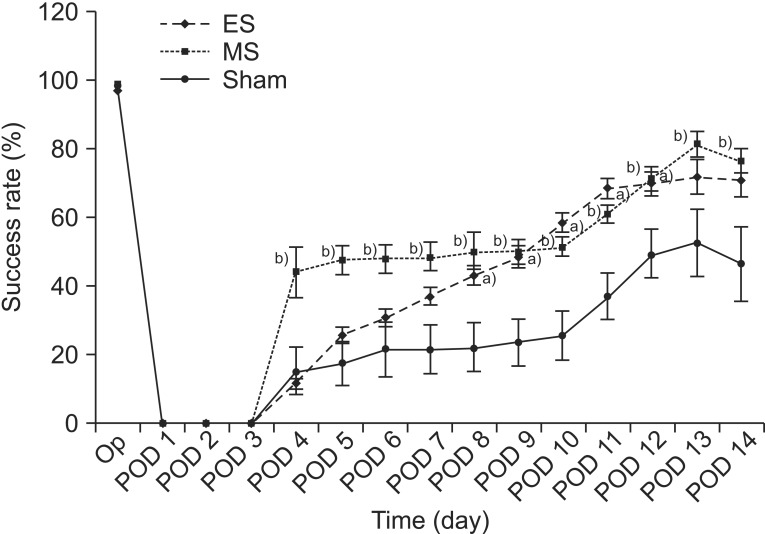
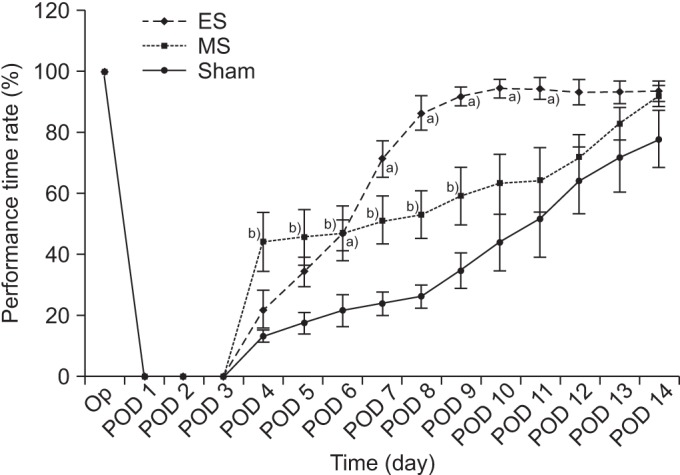
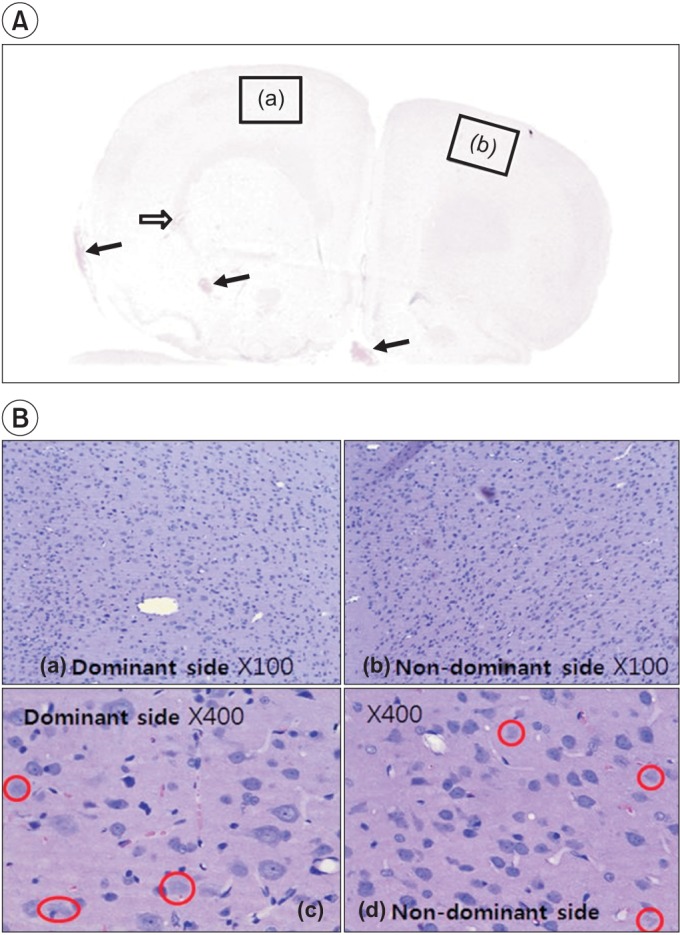
SPRT improved significantly from day 8 to day 12 in the ES group and from day 4 to day 14 in the MS group (p<0.05) compared to the sham group. RRT improved significantly from day 6 to day 11 in ES and from day 4 to day 9 in MS compared to the sham group. The ES and MS groups showed increased expression of c-Fos in the cerebral cortex compared to the sham group.
CONCLUSION
ES or MS in a rat model of diffuse TBI can be used to enhance motor recovery and brain activity.
MeSH Terms
Figure
Reference
-
1. Faul M, Wald MM, Xu L, Coronado VG. National Center for Injury Prevention and Control (U.S.), Division of Injury Response. Traumatic brain injury in the United States: emergency department visits, hospitalizations and deaths 2002-2006 [Internet]. Atlanta: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control;2010. cited 2015 May 15. Abailable from: http://stacks.cdc.gov/view/cdc/5571/.2. Walsh V, Desmond JE, Pascual-Leone A. Manipulating brains. Behav Neurol. 2006; 17:131–134. PMID: 17148832.
Article3. Harvey RL, Nudo RJ. Cortical brain stimulation: a potential therapeutic agent for upper limb motor recovery following stroke. Top Stroke Rehabil. 2007; 14:54–67. PMID: 18174116.
Article4. Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003; 2:145–156. PMID: 12849236.
Article5. Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp Brain Res. 2000; 133:425–430. PMID: 10985677.
Article6. Martin PI, Naeser MA, Theoret H, Tormos JM, Nicholas M, Kurland J, et al. Transcranial magnetic stimulation as a complementary treatment for aphasia. Semin Speech Lang. 2004; 25:181–191. PMID: 15118944.
Article7. Yoon YS, Yu KP, Kim H, Kim HI, Kwak SH, Kim BO. The effect of electric cortical stimulation after focal traumatic brain injury in rats. Ann Rehabil Med. 2012; 36:596–608. PMID: 23185723.
Article8. Marmarou A, Foda MA, van den Brink W, Campbell J, Kita H, Demetriadou K. A new model of diffuse brain injury in rats. Part I: Pathophysiology and biomechanics. J Neurosurg. 1994; 80:291–300. PMID: 8283269.9. Foda MA, Marmarou A. A new model of diffuse brain injury in rats. Part II: Morphological characterization. J Neurosurg. 1994; 80:301–313. PMID: 8283270.10. Ucar T, Tanriover G, Gurer I, Onal MZ, Kazan S. Modified experimental mild traumatic brain injury model. J Trauma. 2006; 60:558–565. PMID: 16531854.
Article11. Kang K, Jeong SW, Chu K, Jung KH, Kim M, Roh JK. Endogenous neural stem cell proliferation after intracerebral hemorrhage in rat model. J Korean Neurol Assoc. 2005; 23:496–502.12. Vergara-Aragon P, Gonzalez CL, Whishaw IQ. A novel skilled-reaching impairment in paw supination on the "good" side of the hemi-Parkinson rat improved with rehabilitation. J Neurosci. 2003; 23:579–586. PMID: 12533618.
Article13. Moon SK, Yang CY, No SE, Kim EY, Lee S, Park SA, et al. Promotion of motor recovery by anodal continuous and low amplitude cortical stimulation in rat stroke model. Lab Anim Res. 2007; 23:25–30.14. Hunter AJ, Mackay KB, Rogers DC. To what extent have functional studies of ischaemia in animals been useful in the assessment of potential neuroprotective agents? Trends Pharmacol Sci. 1998; 19:59–66. PMID: 9550943.
Article15. Bao J, Reier PJ, Munson JB. Enhancement of c-fos expression in neurons of the rat spinal cord after partial denervation: evidence for functional plasticity. Exp Neurol. 1993; 122:189–195. PMID: 8405258.
Article16. Prins ML, Hovda DA. Developing experimental models to address traumatic brain injury in children. J Neurotrauma. 2003; 20:123–137. PMID: 12675967.
Article17. Park HK, Fernandez II, Dujovny M, Diaz FG. Experimental animal models of traumatic brain injury: medical and biomechanical mechanism. Crit Rev Neurosurg. 1999; 9:44–52. PMID: 9933368.
Article18. Barzo P, Marmarou A, Fatouros P, Hayasaki K, Corwin F. Biphasic pathophysiological response of vasogenic and cellular edema in traumatic brain swelling. Acta Neurochir Suppl. 1997; 70:119–122. PMID: 9416297.19. Brown JA, Lutsep H, Cramer SC, Weinand M. Motor cortex stimulation for enhancement of recovery after stroke: case report. Neurol Res. 2003; 25:815–818. PMID: 14669524.
Article20. Brown JA, Lutsep HL, Weinand M, Cramer SC. Motor cortex stimulation for the enhancement of recovery from stroke: a prospective, multicenter safety study. Neurosurgery. 2006; 58:464–473. PMID: 16528186.
Article21. Brown JA, Lutsep HL, Weinand M, Cramer SC. Motor cortex stimulation for the enhancement of recovery from stroke: a prospective, multicenter safety study. Neurosurgery. 2008; 62(Suppl 2):853–862. PMID: 18596422.
Article22. Kim HI, Shin YI, Moon SK, Chung GH, Lee MC, Kim HG. Unipolar and continuous cortical stimulation to enhance motor and language deficit in patients with chronic stroke: report of 2 cases. Surg Neurol. 2008; 69:77–80. PMID: 17825377.
Article23. Adkins-Muir DL, Jones TA. Cortical electrical stimulation combined with rehabilitative training: enhanced functional recovery and dendritic plasticity following focal cortical ischemia in rats. Neurol Res. 2003; 25:780–788. PMID: 14669519.
Article24. Teskey GC, Flynn C, Goertzen CD, Monfils MH, Young NA. Cortical stimulation improves skilled forelimb use following a focal ischemic infarct in the rat. Neurol Res. 2003; 25:794–800. PMID: 14669521.
Article25. Kleim JA, Bruneau R, VandenBerg P, MacDonald E, Mulrooney R, Pocock D. Motor cortex stimulation enhances motor recovery and reduces peri-infarct dysfunction following ischemic insult. Neurol Res. 2003; 25:789–793. PMID: 14669520.
Article26. Plautz EJ, Barbay S, Frost SB, Friel KM, Dancause N, Zoubina EV, et al. Post-infarct cortical plasticity and behavioral recovery using concurrent cortical stimulation and rehabilitative training: a feasibility study in primates. Neurol Res. 2003; 25:801–810. PMID: 14669522.
Article27. Ji RR, Schlaepfer TE, Aizenman CD, Epstein CM, Qiu D, Huang JC, et al. Repetitive transcranial magnetic stimulation activates specific regions in rat brain. Proc Natl Acad Sci U S A. 1998; 95:15635–15640. PMID: 9861022.
Article28. Post A, Muller MB, Engelmann M, Keck ME. Repetitive transcranial magnetic stimulation in rats: evidence for a neuroprotective effect in vitro and in vivo. Eur J Neurosci. 1999; 11:3247–3254. PMID: 10510188.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Application of Non-invasive Brain Stimulation on Dysphagia after Stroke
- Transcranial Electrical Stimulation: Clinical Implication and Practice for Treatment of Psychiatric Illness
- Safety Review for Clinical Application of Repetitive Transcranial Magnetic Stimulation
- Non-Invasive Neuromodulation for Tinnitus
- Electroceutical and Bioelectric Therapy: Its Advantages and Limitations