J Korean Soc Clin Pharmacol Ther.
2012 Dec;20(2):125-134.
Tolerability and Pharmacokinetics Following a Single Dose of Vardenafil in Healthy Korean Volunteers
- Affiliations
-
- 1Department of Clinical Pharmacology and Therapeutics, Seoul National University College of Medicine and Hospital, Seoul, South Korea. ksyu@snu.ac.kr
- 2Department of Clinical Pharmacology and Therapeutics, University of Ulsan and Asan Medical Center, Seoul, South Korea.
Abstract
- BACKGROUND
Vardenafil is a phosphodiesterase type 5 inhibitor, used in erectile dysfunction. This study aimed to evaluate the pharmacokinetics and tolerability of vardenafil following a single oral administration in healthy male subjects.
METHODS
A randomized, double-blind, placebo-controlled, single dosing, dose-escalation study was conducted in 30 healthy subjects. A single oral dose of vardenafil or placebo was given to 10 subjects (8 active + 2 placebo) in each dose group of 5, 10 and 20 mg. Serial blood and urine samples were obtained up to 48 hours for pharmacokinetic analysis. Vardenafil and its metabolite were detected by high performance liquid chromatography tandem mass spectrometry assay.
RESULTS
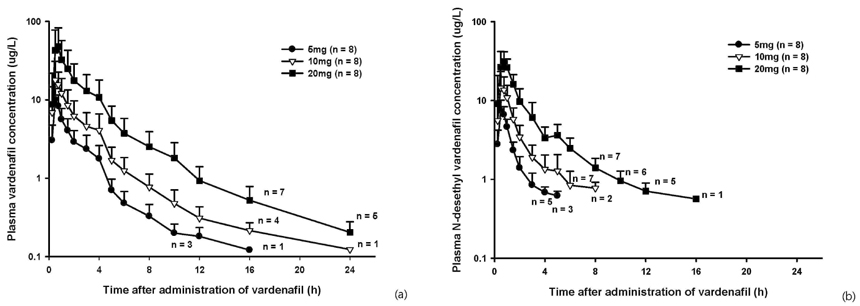
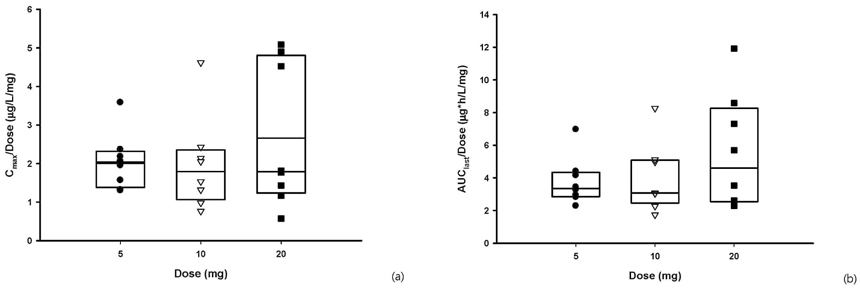
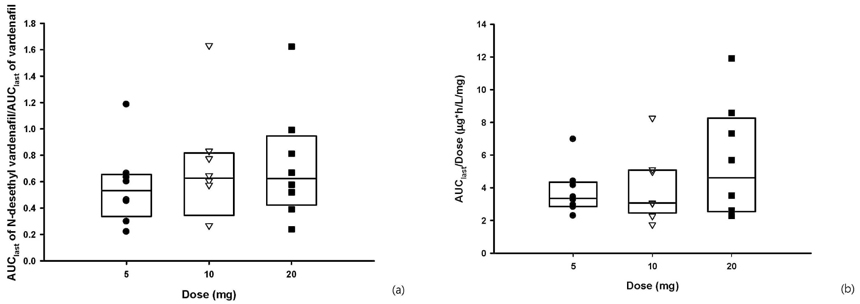
A total of 45 adverse events (AE) were reported in 22 subjects, including 5 AEs from placebo treatment, and all the AEs were mild, except one case of moderate nasal stuffiness. Vardenafil was absorbed after a single oral dose, with the tmax of 0.5-1.0 hours. The Cmax and AUClast were 10.21 +/- 3.68 ug/L(mean +/- SD) and 18.08 +/- 7.44 ugxh/L in 5 mg dose group, 19.79 +/- 12.13 ug/L and 38.61 +/- 21.04 ugxh/L in 10 mg dose group and 53.16 +/- 37.01 ug/L and 110.05 +/- 69.65 ugxh/L in 20 mg dose group. Dose-linearity on AUClast and Cmax of vardenafil were observed in three dose groups. In all dose groups, the fraction excreted in urine was less than 1%.
CONCLUSION
The vardenafil was tolerable over a single dose range of 5 - 20 mg. The pharmacokinetics of vardenfil after a single oral dose was explored and linear pharmacokinetic characteristics were observed over the dose range of 5 - 20 mg in healthy subjects.
MeSH Terms
Figure
Reference
-
1. Lue TF, Giuliano F, Montorsi F, Rosen RC, Andersson KE, Althof S, Christ G, Hatzichristou D, Hirsch M, Kimoto Y, Lewis R, McKenna K, MacMahon C, Morales A, Mulcahy J, Padma-Nathan H, Pryor J, de Tejada IS, Shabsigh R, Wagner G. Summary of the recommendations on sexual dysfunctions in men. J Sex Med. 2004. 1(1):6–23.
Article2. Albersen M, Orabi H, Lue TF. Evaluation and treatment of erectile dysfunction in the aging male: a mini-review. Gerontology. 2012. 58(1):3–14.
Article3. Hatzimouratidis K, Amar E, Eardley I, Giuliano F, Hatzichristou D, Montorsi F, Vardi Y, Wespes E. Guidelines on male sexual dysfunction: erectile dysfunction and premature ejaculation. Eur Urol. 2010. 57(5):804–814.
Article4. Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the US. Am J Med. 2007. 120(2):151–157.5. Thompson IM, Tangen CM, Goodman PJ, Probstfield JL, Moinpour CM, Coltman CA. Erectile dysfunction and subsequent cardiovascular disease. JAMA. 2005. 294(23):2996–3002.6. Cho BL, Kim YS, Choi YS, Hong MH, Seo HG, Lee SY, Shin HC, Kim CH, Moon YS, Cha HS, Kim BS. Prevalence and risk factors for erectile dysfunction in primary care: results of a Korean study. Int J Impot Res. 2003. 15(5):323–328.
Article7. Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994. 151(1):54–61.
Article8. Gupta M, Kovar A, Meibohm B. The clinical pharmacokinetics of phosphodiesterase-5 inhibitors for erectile dysfunction. J Clin Pharmacol. 2005. 45(9):987–1003.
Article9. Research CfDEa. NDA 021400 Levitra (Vardenafil Hydrochloride) Tablets: Clinical Pharmacology/Biopharmaceutics Review. 2003. Rockville, Md: Department of Health and Human Services, US Food and Drug Administration.10. EMEA. Scientific discussion for the approval of Levitra [Online]. last visited on 27 May 2012.11. Shon JH, Ku HY, Bae SY, Oh MK, Yeo CW, Bae SK, Shin JG. The disposition of three phosphodiesterase type 5 inhibitors, vardenafil, sildenafil, and udenafil, is differently influenced by the CYP3A5 genotype. Pharmacogenet Genomics. 2011. 21(12):820–828.
Article12. Dorne JL, Walton K, Renwick AG. Human variability in CYP3A4 metabolism and CYP3A4-related uncertainty factors for risk assessment. Food Chem Toxicol. 2003. 41(2):201–224.
Article13. Park SY, Kang YS, Jeong MS, Yoon HK, Han KO. Frequencies of CYP3A5 genotypes and haplotypes in a Korean population. J Clin Pharm Ther. 2008. 33(1):61–65.
Article14. Choi MK, Song IS. Characterization of efflux transport of the PDE5 inhibitors, vardenafil and sildenafil. J Pharm Pharmacol. 2012. 64(8):1074–1083.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pharmacokinetics of T-614 after Single Oral Administration in Healthy Korean Volunteers
- Pharmacokinetics and Tolerability Evaluation of Fudosteine after Oral Administration in Healthy Korean Volunteers
- A Randomized, Placebo Controlled, Double Blind, Parallel Group, Multiple Dosing, Dose Escalation Clinical Study to Evaluate Pharmacokinetics/Pharmacodynamics and Tolerability of Zofenopril in Healthy Korean Subjects
- Pharmacokinetic Characteristics of Ibandronate and Tolerability of DP-R206 (150 mg Ibandronate/24,000 IU Vitamin D3) Compared to the Ibandronate (150 mg) Monotherapy in Healthy Adults
- Pharmacokinetics, Efficacy, and Safety of Selective Inhibitors of Phosphodiesterase Type 5 and Sublingual Apomorphine for the Treatment of Erectile Dysfunction