J Korean Diabetes Assoc.
2006 Jan;30(1):54-63.
The effects of mixed chimerism conducted by natural killer cell depletion with non myeloablation on islet allograft rejection
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Division of Hemato-Oncology, The Catholic University of Korea.
- 2Department of Internal Medicine, The Catholic University of Korea.
- 3Department of Microbiology and Immunology, Tumor Immunity Medical Research Center, Transplantation Research Institute, Seoul National University College of Medicine.
Abstract
-
BACKGROUND: Because of the shortage of human pancreas and immunorejection, very small fraction of patients with type 1 diabetes can be treated with islet transplantation. The immune tolerance induction for overcoming the immume rejectin of trausplamted islets could be conducted by hematopoietic mixed chimerims with various invasive methods. The purpose of this study is to investigate the effect of mixed chimerims conducted by newly developed minimally invasive methods on islet allografts rejection in streptozotocin induced diabetic mice.
METHODS
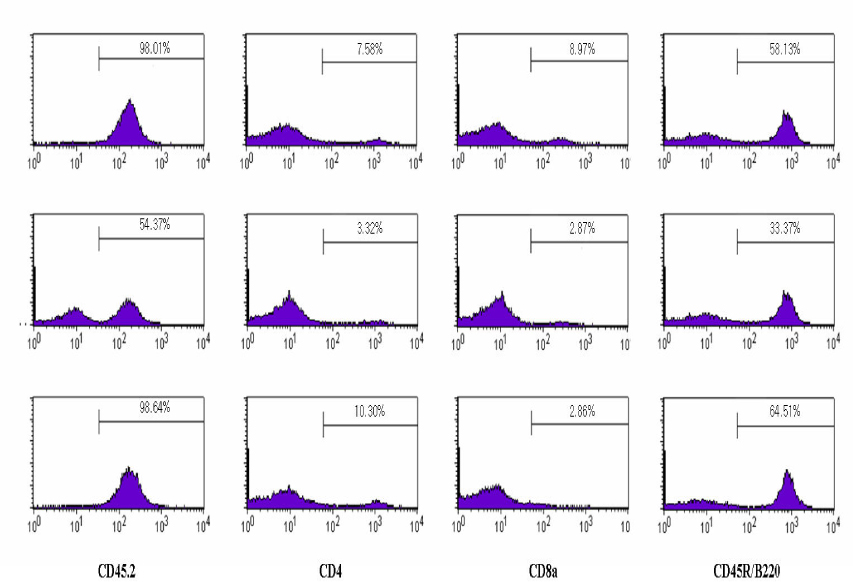
Recipient, Balb/c(H-2Kd) mice were injected intraperitoneally with anti- asialoGM1 antibody at one day before bone marrow transplantation. There were received total body irradiation at a dose of 500 cGy and followed by tail vein injection of the 2 x 10(7) T-cell depleted bone marrow cells from C57BL/6(H-2Kb). Mixed chimerism mice were determined by gDNA PCR of lymphocyte MHC class I gene (H-2K) on 21st day. Streptozotocin induced diabetic mixed chimera mice were received islet transplantation from bone marrow donors. Grafts, spleen and peripheral blood were obtained from the mixed chimera mice, and there were used by Immunohistochimeical staining, flow cytometric analysis and gDNA PCR on 21st day.
RESULTS
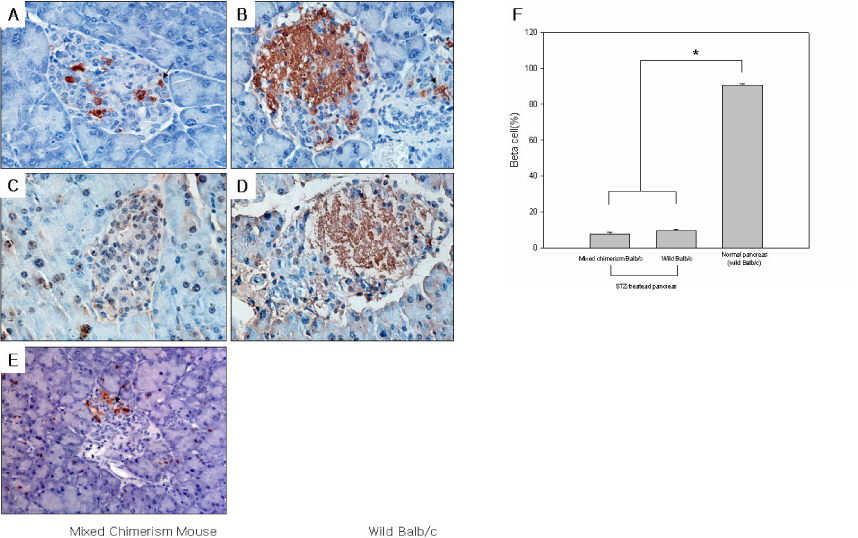
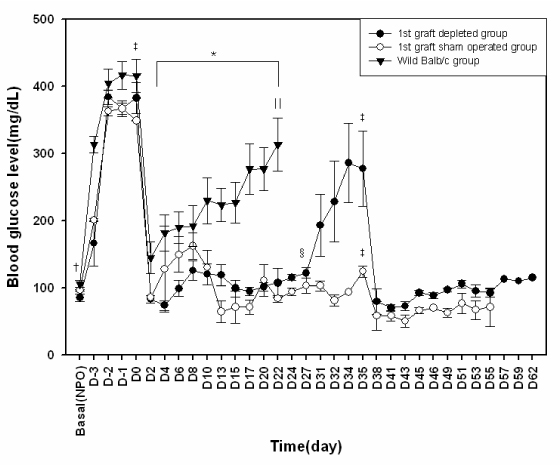
The blood glucose levels of streptozotocin induced diabetic mice were normalized by transplantation of bone marrow donor islets and maintained during 30 days. After removal of first islet allografts, hyperglycemia was re-established. We could re-confirmed donor specific tolerance of transplanted islets by second transplantation of bone marrow donor islets. Normoglycemia was maintained during 21 days after second islet transplantation. Furthermore islet grafts from MHC-mismatched third party mice were immediately rejected. Flow cytometric analysis results suggest that the mixed chimerism mice were maintain during the whole study period.
CONCLUSION
The mixed chimerism model conducted by newly developed and minimally invasive method effectively prevents the islet allo grafts rejection in STZ-induced mixed chimerism mice.
Keyword
MeSH Terms
-
Allografts*
Animals
Blood Glucose
Bone Marrow
Bone Marrow Cells
Bone Marrow Transplantation
Chimera
Chimerism*
Genes, MHC Class I
Humans
Hyperglycemia
Immune Tolerance
Insulin
Islets of Langerhans Transplantation
Killer Cells, Natural*
Lymphocytes
Mice
Pancreas
Polymerase Chain Reaction
Spleen
Streptozocin
T-Lymphocytes
Tissue Donors
Transplantation
Transplants
Veins
Whole-Body Irradiation
Blood Glucose
Insulin
Streptozocin
Figure
Reference
-
1. Atkinson MA, Maclaren NK. The pathogenesis of insulin-dependent diabetes mellitus. N Engl J Med. 1994. 331(21):1428–1436.2. Patricia M Graves, George S Eisenbarth. Pathogenesis, prediction and trials for the prevention of insulin-dependent (type 1) diabetes mellitus. Advanced Drug Delivery Reviews. 1999. 35:143–156.3. Stratta R. Late acute rejection after pancreas transplantation. Transplantation Proceedings. 2000. 30:646.4. Nakhleh RE, Gruessner RWG. Ischemia due to vascular rejection causes islet loss after pancreas transplantation. Transplantation Proceedings. 1998. 30:539–540.5. Spiros Delis, Gaetano Ciancioa C, George W Burke III, Rolando Garcia-Morales, Joshua Miller. Donor bone marrow transplantation chimerism and tolerance. Transplant Immunology. 2004. 13:105–115.6. kolb Hans-jochem, ledderose georg. Tolerance and chimerism. Transplantati. 2003. 75:26s–31s.7. Li Hua, Inverardi Luca, Damaris R Molano, Antonello Pileggi, Camillo Ricordi. Nonlethal condiioning for the induction of allogeneic chimerism and tolerance to islet allografts. Transplantation. 2003. 75:966–970.8. Zhiguang Guo, Tao Wu, Hakan Sozen, Yisheng Pan, Neal Heuss, Hannes Kalscheuer, Sutherland David ER, Blazar Bruce R, Hering Bernhard J. A substantial level of donor hematopoietic chimerism is required to protect donor=specific islet grafts in diabetic nod mice. Transplantation. 2003. 75:909–915.9. Murphy WilliamJ, Kumar Vinay, Bennett Michael. Acute rejection of murine vone marrow allografts by natural killer cells and T cells-Differences in kinetic and Target antigen recognized. J Exp Med. 1987. 166:1499–1509.10. Tiberghien Pierre, Longo Dan L, Wine John W, Alvord W Gregory, Reynolds Craig W. Anti-asialo GM1 antiserum treatment of lethally irradiated recipients before bone marrow transplantation: Evidence that recipient natural killer depletion enhances survival, engraftment, and hematopoietic recovery. Blood. 1990. 76:1419–1430.11. Murphy William J, Koh Crystal Y, Raziuddin Arati, Bennett Michael, Longo Dan L. Immunobiology of natural killer cells and bone marrow transplantation: Merging of basic and preclinical studies. Immunological Reviews. 2001. 181:279–289.12. Cho Seok Goo, Shuto Yukinobu, Soda Yasushi, Nakazaki Yukoh, Izawa Kiyoko, Uchimaru Kaoru, Takahashi Satoshi, Tani Kenzaburo, Tojo Arinobu, Asano Shigetaka. Anti-NK cell treatment induces stable mixed chimerism in MHC-mismatched, T cell-depleted, nonmyeloablative bone marrow transplantation. Exp Hematol. 2004. 32:1246–1254.13. Weibel ER. Stereplogic methods. practical methods for biologic morphometry. 1978. 1. London: Academic Press;101–161.14. Drachenberg Cinthia B, Klassen David K, Weir Matthew R, Wiland Ann, Fink Jeffrey C, Bartlett Stephen T, Cangro Charles B, Blahut Steven, Papadimitriou John C. Islet cell damage associated with tacrolimus and cyclosporine: Morphological features in pancreas allograft biopsieds and clinical correlation. Transplantation. 1999. 68:396–402.15. Guo Zhiguang, Chong Anita SF, Shen Jikun, Foster Preston, Sankary Howard N, McChesney Lawrence, Mital Deepak, Jensik Stephen C, Gebel Howard, Williams James W. In vivo effects of leflunomide on normal pancreatic islet and syngeneic islet graft function. Transplantation. 1997. 63:716–721.16. Russell Paul S, Chase Catharine M, Sykes Megan, Ito Hiroshi, Shaffer Juanita, Colvin Robert B. Tolerance, mixed chimerism, and chronic transplant arteriopathy. J Immunol. 2001. 167:5731–5740.17. Sykes M. Mixed chimerism and transplant tolerance. Immunity. 2001. 14:417–424.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Strategies to Develop Transplantation Tolerance
- Prevention of Allograft Rejection by Immune Tolerance
- Lineage-specific chimerism analysis in nucleated cells, T cells and natural killer cells after myeloablative allogeneic hematopoietic stem cell transplantation
- Role of Regulatory T Cells in Transferable Immunological Tolerance to Bone Marrow Donor in Murine Mixed Chimerism Model
- Spontaneous diabetes mellitus in the BB rat: effect of depletion ofT lymphocytes and natural killer cells on anti-islet cellular cytotoxicity