Korean J Urol.
2010 Nov;51(11):794-799.
Effect of Korean Red Ginseng on Testicular Tissue Injury after Torsion and Detorsion
- Affiliations
-
- 1Department of Urology, Chungnam National University School of Medicine, Daejeon, Korea. uro17@cnuh.co.kr
- 2Department of Life Science, Korea Basic Science Institute (KBSI), Daejeon, Korea.
Abstract
- PURPOSE
Korean red ginseng (KRG) is a potent antioxidant and a free radical scavenger. This study was designed to determine whether KRG could protect against dysfunction and oxidative stress induced by torsion-detorsion injury in rat testis.
MATERIALS AND METHODS
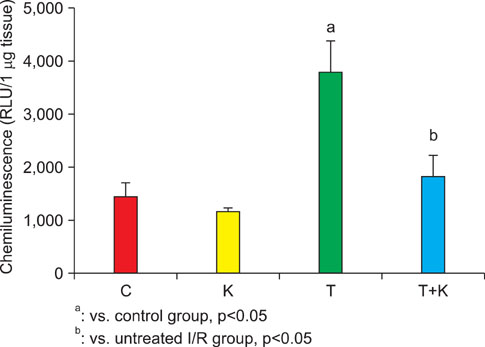
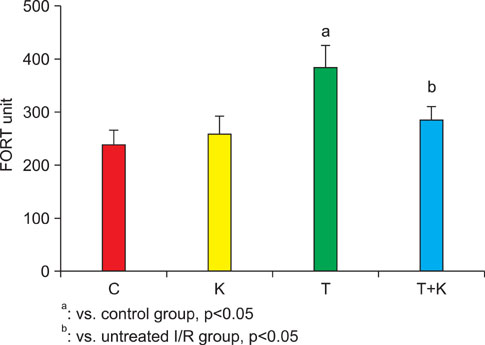
Six-week-old male Sprague-Dawley rats were randomly divided into four groups: a sham-operated control group (C), a sham-operated and KRG-treated group (K), a 2 hours torsion and detorsion group (T), and a 2 hours torsion and detorsion and KRG-treated group (T+K). We measured testis weight and hormone levels and reactive oxygen species (ROS) from the left renal vein. Superoxide generation was measured on the basis of lucigenin-enhanced chemiluminescence in testis tissue.
RESULTS
Testicular weight was significantly higher in the T+K group than in the T group; however, there were no significant differences in hormone levels between the 4 groups. The mean level of ROS and superoxide production was significantly higher in the T group than in the C group, whereas administration of KRG attenuated this increase. Upon histologic evaluation, the T group was found to have cellular disarray, a lack of cellular cohesiveness, degenerative changes in the germinal cells, and less distinct changes in the seminiferous tubules, whereas the T+K group had a germinal epithelial layer that appeared nearly normal.
CONCLUSIONS
The present study demonstrated that KRG recovered the testis dysfunction in the rat testis by suppressing superoxide production.
Keyword
MeSH Terms
Figure
Reference
-
1. Barada JH, Weingarten JL, Cromie WJ. Testicular salvage and age-related delay in the presentation of testicular torsion. J Urol. 1989. 142:746–748.2. Cattolica EV, Karol JB, Rankin KN, Klein RS. High testicular salvage rate in torsion of the spermatic cord. J Urol. 1982. 128:66–68.3. Wei SM, Yan ZZ, Zhou J. Beneficial effect of taurine on testicular ischemia-reperfusion injury in rats. Urology. 2007. 70:1237–1242.4. Unsal A, Devrim E, Guven C, Eroglu M, Durak I, Bozoklu A, et al. Propofol attenuates reperfusion injury after testicular torsion and detorsion. World J Urol. 2004. 22:461–465.5. Power RE, Scanlon R, Kay EW, Creagh TA, Bouchier-Hayes DJ. Long-term protective effects of hypothermia on reperfusion injury post-testicular torsion. Scand J Urol Nephrol. 2003. 37:456–460.6. Sugaya A, Yuzurihara M, Tsuda T, Yasuda K, Kajiwara K, Sugaya E. Proliferative effect of ginseng saponin on neurite extension of primary cultured neurons of the rat cerebral cortex. J Ethnopharmacol. 1988. 22:173–181.7. Hasegawa H, Suzuki R, Nagaoka T, Tezuka Y, Kadota S, Saiki I. Prevention of growth and metastasis of murine melanoma through enhanced natural-killer cytotoxicity by fatty acid-conjugate of protopanaxatriol. Biol Pharm Bull. 2002. 25:861–866.8. Kaneko H, Nakanishi K. Proof of the mysterious efficacy of ginseng: basic and clinical trials: clinical effects of medical ginseng, Korean red ginseng: specifically, its anti-stress action for prevention of disease. J Pharmacol Sci. 2004. 95:158–162.9. Chen X. Cardiovascular protection by ginsenosides and their nitric oxide releasing action. Clin Exp Pharmacol Physiol. 1996. 23:728–732.10. Zhu D, Wu L, Li CR, Wang XW, Ma YJ, Zhong ZY, et al. Ginsenoside Rg1 protects rat cardiomyocyte from hypoxia/reoxygenation oxidative injury via antioxidant and intracellular calcium homeostasis. J Cell Biochem. 2009. 108:117–124.11. Maffei Facino R, Carini M, Aldini G, Berti F, Rossoni G. Panax ginseng administration in the rat prevents myocardial ischemia-reperfusion damage induced by hyperbaric oxygen: evidence for an antioxidant intervention. Planta Med. 1999. 65:614–619.12. Voces J, Alvarez AI, Vila L, Ferrando A, Cabral de Oliveira C, Prieto JG. Effects of administration of the standardized Panax ginseng extract G115 on hepatic antioxidant function after exhaustive exercise. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1999. 123:175–184.13. Voces J, Cabral de Oliveira AC, Prieto JG, Vila L, Perez AC, Duarte ID, et al. Ginseng administration protects skeletal muscle from oxidative stress induced by acute exercise in rats. Braz J Med Biol Res. 2004. 37:1863–1871.14. Kaneko H, Nakanishi K. Proof of the mysterious efficacy of ginseng: basic and clinical trials: clinical effects of medical ginseng, korean red ginseng: specifically, its anti-stress action for prevention of disease. J Pharmacol Sci. 2004. 95:158–162.15. Kim CS, Park JB, Kim KJ, Chang SJ, Ryoo SW, Jeon BH. Effect of Korea red ginseng on cerebral blood flow and superoxide production. Acta Pharmacol Sin. 2002. 23:1152–1156.16. Skatchkov MP, Sperling D, Hink U, Mülsch A, Harrison DG, Sindermann I, et al. Validation of lucigenin as a chemiluminescent probe to monitor vascular superoxide as well as basal vascular nitric oxide production. Biochem Biophys Res Commun. 1999. 254:319–324.17. Jeon BH, Gupta G, Park YC, Qi B, Haile A, Khanday FA, et al. Apurinic/apyrimidinic endonuclease 1 regulates endothelial NO production and vascular tone. Circ Res. 2004. 95:902–910.18. Yazihan N, Ataoglu H, Koku N, Erdemli E, Sargin AK. Protective role of erythropoietin during testicular torsion of the rats. World J Urol. 2007. 25:531–536.19. Horica CA, Hadziselimovic F, Kreutz G, Bandhauer K. Ultrastructural studies of the contorted and contralateral testicle in unilateral testicular torsion. Eur Urol. 1982. 8:358–362.20. Thomas WE, Williamson RC. Diagnosis and outcome of testicular torsion. Br J Surg. 1983. 70:213–216.21. Unsal A, Eroglu M, Avci A, Cimentepe E, Guven C, Derya Balbay M, et al. Protective role of natural antioxidant supplementation on testicular tissue after testicular torsion and detorsion. Scand J Urol Nephrol. 2006. 40:17–22.22. Cay A, Alver A, Kücük M, Isik O, Eminagaoglu MS, Karahan SC, et al. The effects of N-acetylcysteine on antioxidant enzyme activities in experimental testicular torsion. J Surg Res. 2006. 131:199–203.23. Lim DJ, Hong SK, Jeong SJ, Choi H. The mechanism of damage to the contralateral testis following testicular torsion and detorsion in rats and the effect of allopurinol administration. Korean J Urol. 2006. 47:180–188.24. Kitts DD, Wijewickreme AN, Hu C. Antioxidant properties of a North American ginseng extract. Mol Cell Biochem. 2000. 203:1–10.25. Kim YK, Guo Q, Packer L. Free radical scavenging activity of red ginseng aqueous extracts. Toxicology. 2002. 172:149–156.26. Siddique MS, Eddeb F, Mantle D, Mendelow AD. Extracts of Ginkgo biloba and Panax ginseng protect brain proteins from free radical induced oxidative damage in vitro. Acta Neurochir Suppl. 2000. 76:87–90.27. Granger DN, Korthuis RJ. Physiologic mechanisms of postischemic tissue injury. Annu Rev Physiol. 1995. 57:311–332.28. Mercuro G, Cadeddu C, Piras A, Dessi M, Madeddu C, Deidda M, et al. Early epirubicin-induced myocardial dysfunction revealed by serial tissue Doppler echocardiography: correlation with inflammatory and oxidative stress markers. Oncologist. 2007. 12:1124–1133.29. Abramson JL, Hooper WC, Jones DP, Ashfaq S, Rhodes SD, Weintraub WS, et al. Association between novel oxidative stress markers and C-reactive protein among adults without clinical coronary heart disease. Atherosclerosis. 2005. 178:115–121.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effect of testicular torsion on the contralateral testis in rats
- The Effect of Free Radical Scavengers on Reperfusion Injury after Testicular Torsion
- The Mechanism of Damage to the Contralateral Testis Following Testicular Torsion and Detorsion in Rats and the Effect of Allopurinol Administration
- Clinical Significance of Testicular Biopsy in Patient of Spermatic Cord Torsion
- The Effect of Testicular Torsion on the Histologic Findings and Apoptosis of the Contralateral Testis at Various Ages in Rats