Nutr Res Pract.
2015 Jun;9(3):227-234. 10.4162/nrp.2015.9.3.227.
Dual effects of a mixture of grape pomace (Campbell Early) and Omija fruit ethanol extracts on lipid metabolism and the antioxidant defense system in diet-induced obese mice
- Affiliations
-
- 1Department of Food Science and Nutrition, Kyungpook National University, 80 Daehakro, Bukgu, Daegu 702-701, Korea. mschoi@knu.ac.kr
- 2Center for Food and Nutritional Genomics Research, Kyungpook National University, Daegu 702-701, Korea.
- 3Food R&D, CJ Cheiljedang Corp., Seoul 152-051, Korea.
- 4School of Life Science & Biotechnology, Kyungpook National University, Daegu 702-701, Korea.
- KMID: 2313834
- DOI: http://doi.org/10.4162/nrp.2015.9.3.227
Abstract
- BACKGROUND/OBJECTIVES
We investigated the effects of a combination of grape pomace (Vitis labrusca, Campbell Early) and Omija fruit (Schizandra chinensis, Baillon) ethanol extracts on lipid metabolism and antioxidant defense system in diet-induced obese mice.
MATERIALS/METHODS
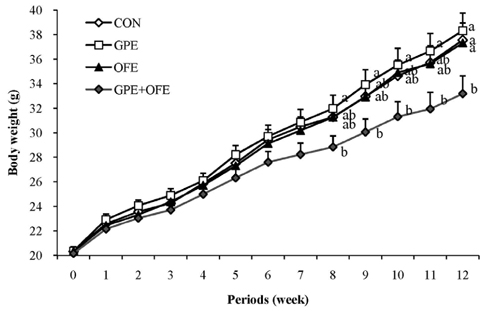
Forty male C57BL/6J mice were divided into four groups and fed high-fat diet (control group, CON) or high-fat diet added 0.5% grape pomace extract (GPE), 0.05% Omija fruit extract (OFE) or 0.5% GPE plus 0.05% OFE (GPE+OFE) for 12 weeks.
RESULTS
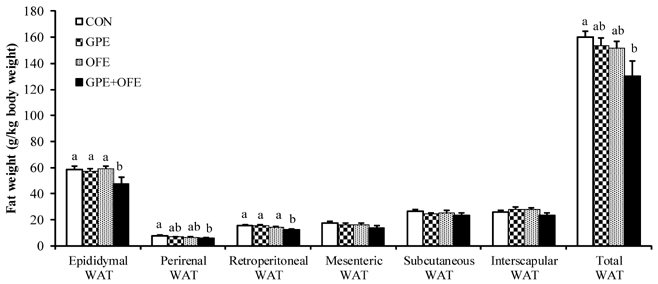
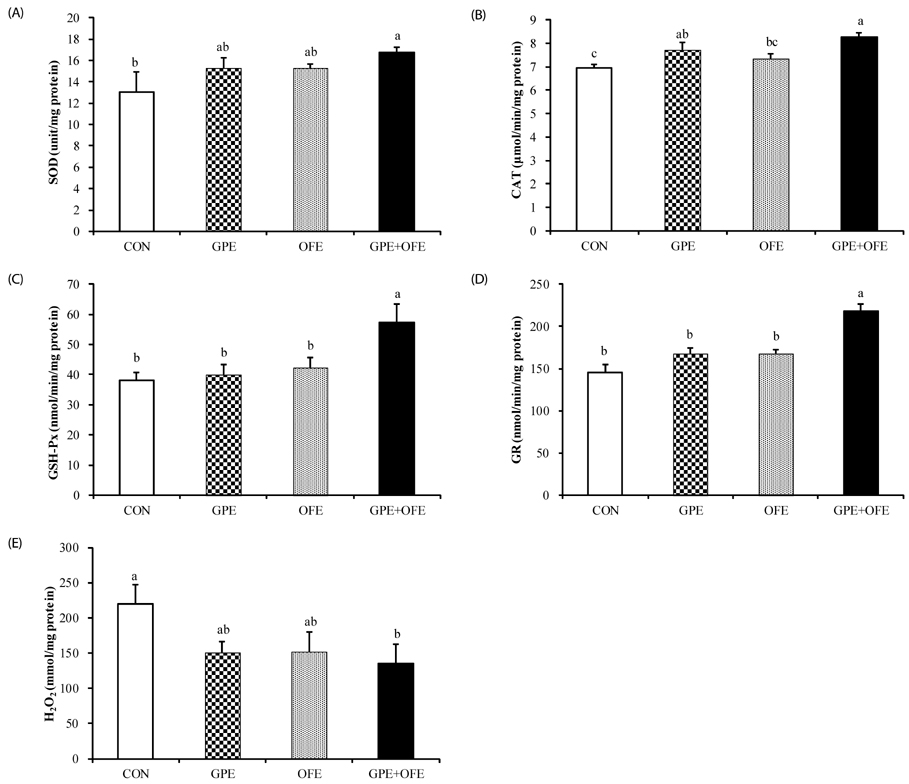
In contrast to the GPE- or OFE-supplemented groups, the GPE+OFE group showed significantly lower body weight and white adipose tissue weights than the CON group. Moreover, GPE+OFE supplementation significantly decreased plasma total cholesterol and increased the plasma HDL-cholesterol/total-cholesterol ratio (HTR) compared to the control diet. The hepatic triglyceride level was significantly lower in the GPE+OFE and GPE groups by increasing beta-oxidation and decreasing lipogenic enzyme compared to the CON group. Furthermore, GPE+OFE supplementation significantly increased antioxidant enzyme activities with a simultaneous decrease in liver H2O2 content compared to the control diet.
CONCLUSIONS
Together our results suggest that supplementation with the GPE+OFE mixture may be more effective in improving adiposity, lipid metabolism and oxidative stress in high-fat diet-fed mice than those with GPE and OFE alone.
MeSH Terms
Figure
Reference
-
1. Kopelman PG. Obesity as a medical problem. Nature. 2000; 404:635–643.
Article2. Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010; 51:679–689.
Article3. Bray GA, Tartaglia LA. Medicinal strategies in the treatment of obesity. Nature. 2000; 404:672–677.
Article4. Yun JW. Possible anti-obesity therapeutics from nature--a review. Phytochemistry. 2010; 71:1625–1641.
Article5. Liu RH. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J Clin Nutr. 2003; 78:517S–520S.
Article6. Kota SK, Jammula S, Kota SK, Satya Krishna SV, Meher LK, Rao ES, Modi KD. Nutraceuticals in pathogenic obesity; striking the right balance between energy imbalance and inflammation. J Med Nutr Nutraceuticals. 2012; 1:63–76.
Article7. Gehm BD, McAndrews JM, Chien PY, Jameson JL. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc Natl Acad Sci USA. 1997; 94:14138–14143.
Article8. Pastrana-Bonilla E, Akoh CC, Sellappan S, Krewer G. Phenolic content and antioxidant capacity of muscadine grapes. J Agric Food Chem. 2003; 51:5497–5503.
Article9. Makris DP, Boskou G, Andrikopoulos NK, Kefalas P. Characterisation of certain major polyphenolic antioxidants in grape (Vitis vinifera cv. Roditis) stems by liquid chromatography-mass spectrometry. Eur Food Res Technol. 2008; 226:1075–1079.
Article10. Hogan S, Canning C, Sun S, Sun X, Zhou K. Effects of grape pomace antioxidant extract on oxidative stress and inflammation in diet induced obese mice. J Agric Food Chem. 2010; 58:11250–11256.
Article11. Saunders RM. Monograph of Kadsura (Schisandraceae). Syst Bot Monogr. 1998; 54:1–106.
Article12. Lin Q, Duan L, Yao B. Notes on three species of the genus Kadsura Juss. (Schisandraceae). Acta Phytotaxonomica Sinica. 2005; 43:567–570.
Article13. Zhang CL, He XL. Advances in research on Schisandra chinesis (Turcz.) Baill. J Baoding Teach Coll. 2005; 17:36–39.14. Kim SH, Joo MH, Yoo SH. Structural identification and antioxidant properties of major anthocyanin extracted from Omija (Schizandra chinensis) fruit. J Food Sci. 2009; 74:C134–C140.
Article15. Guo LY, Hung TM, Bae KH, Shin EM, Zhou HY, Hong YN, Kang SS, Kim HP, Kim YS. Anti-inflammatory effects of schisandrin isolated from the fruit of Schisandra chinensis Baill. Eur J Pharmacol. 2008; 591:293–299.
Article16. Park HJ, Cho JY, Kim MK, Koh PO, Cho KW, Kim CH, Lee KS, Chung BY, Kim GS, Cho JH. Anti-obesity effect of Schisandra chinensis in 3T3-L1 cells and high fat diet-induced obese rats. Food Chem. 2012; 134:227–234.
Article17. Choi SY, Lee Y, Lee P, Kim KT. Comparison of the antioxidative effects and content of anthocyanin and phenolic compounds in different varieties of Vitis vinifera ethanol extract. J Food Sci Nutr. 2011; 16:24–28.
Article18. Cho SJ, Jung UJ, Park HJ, Kim HJ, Park YB, Kim SR, Choi MS. Combined ethanol extract of grape pomace and Omija fruit ameliorates adipogenesis, hepatic steatosis, and inflammation in diet-induced obese mice. Evid Based Complement Alternat Med. 2013; 2013:212139.
Article19. Cho SJ, Jung UJ, Choi MS. Differential effects of low-dose resveratrol on adiposity and hepatic steatosis in diet-induced obese mice. Br J Nutr. 2012; 108:2166–2175.
Article20. Hulcher FH, Oleson WH. Simplified spectrophotometric assay for microsomal 3-hydroxy-3-methylglutaryl CoA reductase by measurement of coenzyme A. J Lipid Res. 1973; 14:625–631.
Article21. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957; 226:497–509.
Article22. Ochoa S. Malic enzyme: malic enzymes from pigeon and wheat germ. Methods Enzymol. 1995; 1:323–326.23. Pitkänen E, Pitkänen O, Uotila L. Enzymatic determination of unbound D-mannose in serum. Eur J Clin Chem Clin Biochem. 1997; 35:761–766.
Article24. Nepokroeff CM, Lakshmanan MR, Porter JW. Fatty-acid synthase from rat liver. Methods Enzymol. 1975; 35:37–44.25. Walton PA, Possmayer F. Mg2-dependent phosphatidate phosphohydrolase of rat lung: development of an assay employing a defined chemical substrate which reflects the phosphohydrolase activity measured using membrane-bound substrate. Anal Biochem. 1985; 151:479–486.
Article26. Lazarow PB. Assay of peroxisomal β-oxidation of fatty acids. Methods Enzymol. 1981; 72:315–319.27. Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974; 47:469–474.
Article28. Aebi H. Catalase. In : Bergmeyer HU, Gawehn K, editors. Methods of Enzymatic Analysis. 2nd ed. Weinheim: Academic Press;1974. p. 673–684.29. Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967; 70:158–169.30. Pinto RE, Bartley W. The effect of age and sex on glutathione reductase and glutathione peroxidase activities and on aerobic glutathione oxidation in rat liver homogenates. Biochem J. 1969; 112:109–115.
Article31. Wolff SP. Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Methods Enzymol. 1994; 233:182–189.32. Yunoki K, Sasaki G, Tokuji Y, Kinoshita M, Naito A, Aida K, Ohnishi M. Effect of dietary wine pomace extract and oleanolic acid on plasma lipids in rats fed high-fat diet and its DNA microarray analysis. J Agric Food Chem. 2008; 56:12052–12058.
Article33. Khanal RC, Howard LR, Rogers TJ, Wilkes SE, Dhakal IB, Prior RL. Effect of feeding grape pomace on selected metabolic parameters associated with high fructose feeding in growing Sprague-Dawley rats. J Med Food. 2011; 14:1562–1569.
Article34. Samra JS. Sir David Cuthbertson Medal Lecture. Regulation of lipid metabolism in adipose tissue. Proc Nutr Soc. 2000; 59:441–446.35. Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990; 12:1106–1110.
Article36. Brown CD, Higgins M, Donato KA, Rohde FC, Garrison R, Obarzanek E, Ernst ND, Horan M. Body mass index and the prevalence of hypertension and dyslipidemia. Obes Res. 2000; 8:605–619.
Article37. Lu W, Resnick HE, Jablonski KA, Jones KL, Jain AK, Howard WJ, Robbins DC, Howard BV. Non-HDL cholesterol as a predictor of cardiovascular disease in type 2 diabetes: the strong heart study. Diabetes Care. 2003; 26:16–23.
Article38. Loftus TM, Jaworsky DE, Frehywot GL, Townsend CA, Ronnett GV, Lane MD, Kuhajda FP. Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science. 2000; 288:2379–2381.
Article39. Park J, Rho HK, Kim KH, Choe SS, Lee YS, Kim JB. Overexpression of glucose-6-phosphate dehydrogenase is associated with lipid dysregulation and insulin resistance in obesity. Mol Cell Biol. 2005; 25:5146–5157.
Article40. Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004; 114:1752–1761.
Article41. Betteridge DJ. What is oxidative stress? Metabolism. 2000; 49:3–8.
Article42. Lee SJ, Choi SK, Seo JS. Grape skin improves antioxidant capacity in rats fed a high fat diet. Nutr Res Pract. 2009; 3:279–285.
Article43. Kim JS, Choi SY. Physicochemical properties and antioxidative activities of Omija (Schizandra chinensis Bailon). Korean J Food Nutr. 2008; 21:35–42.44. Tsuda T. Regulation of adipocyte function by anthocyanins; possibility of preventing the metabolic syndrome. J Agric Food Chem. 2008; 56:642–646.
Article45. Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell. 2006; 127:1109–1122.
Article46. Kim S, Jin Y, Choi Y, Park T. Resveratrol exerts anti-obesity effects via mechanisms involving down-regulation of adipogenic and inflammatory processes in mice. Biochem Pharmacol. 2011; 81:1343–1351.
Article47. Cho SJ, Park HJ, Jung UJ, Kim HJ, Moon BS, Choi MS. The beneficial effects of combined grape pomace and Omija fruit extracts on hyperglycemia, adiposity and hepatic steatosis in db/db mice: a comparison with major index compounds. Int J Mol Sci. 2014; 15:17778–17789.
Article48. Drotman RB, Lawhorn GT. Serum enzymes as indicators of chemically induced liver damage. Drug Chem Toxicol. 1978; 1:163–171.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Dietary Grape Pomace on Lipid Oxidation and Related Enzyme Activities in Rats Fed High Fat Diet
- Effects of grape pomace on the antioxidant defense system in diet-induced hypercholesterolemic rabbits
- Effect of Grape Intake on Cadmium Metabolism of Rats during Aging
- Antimicrobial Activities of Omija Extracts Against Bacillus cereus and Escherchia coli
- Grape skin improves antioxidant capacity in rats fed a high fat diet