Nutr Res Pract.
2014 Jun;8(3):249-256.
Effects of plant-based Korean food extracts on lipopolysaccharide-stimulated production of inflammatory mediators in vitro
- Affiliations
-
- 1Department of Nutritional Science and Food Management, Ewha Womans University, 52, Ewhayeodae-gil, Seodaemun-gu, Seoul 120-750, Korea. yuri.kim@ewha.ac.kr
- 2Department of Food and Nutrition, Hannam University, Daedeok Valley Campus, Daejeon 305-811, Korea.
- 3Department of Food and Nutrition, Kyung Hee University, Seoul 130-701, Korea.
Abstract
- BACKGROUND/OBJECTIVES
The traditional Korean diet is plant-based and rich in antioxidants. Previous studies have investigated the potential health benefits of individual nutrients of Korean foods. However, the cumulative effects of a Korean diet on inflammation remain poorly understood. Therefore, the aim of this study was to investigate the anti-inflammatory effects of a plant-based Korean diet.
MATERIALS/METHODS
Using data from the Fifth Korean National Health and Nutrition Examination Survey, 75 individual plant food items were selected which represent over 1% of the total diet intake of the Korean diet. These items were classified into ten different food groups, and the vegetable (Veg) and fruit (Fruit) groups were studied based on their high antioxidant capacity. For comparison, a mixture of all ten groups (Mix) was prepared. To produce a model of inflammation with which to test these Veg, Fruit, and Mix plant-based Korean food extracts (PKE), RAW264.7 macrophages were treated with lipopolysaccharide (LPS).
RESULTS
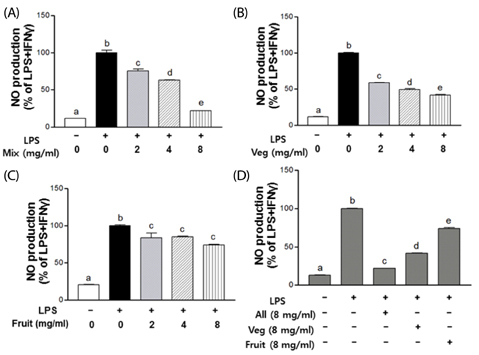
Levels of nitric oxide (NO) and prostaglandin E2 (PGE2), as well as protein expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) were found to be lower following PKE treatment. Furthermore, PKE treatment was found to suppress tumor necrosis factor-alpha (TNF-alpha) and interleukin-6 (IL-6) via the nuclear transcription factor kappa-B (NF-kappaB) signaling pathway. Overall, the Mix group exhibited the greatest anti-inflammatory effects compared with Veg and Fruit PKE group.
CONCLUSIONS
Inhibition of LPS-induced pro-inflammatory mediators by the PKE tested was found to involve an inhibition of NF-kB activation. Moreover, PKE tested have the potential to ameliorate various inflammation-related diseases by limiting the excessive production of pro-inflammatory mediators.
Keyword
MeSH Terms
-
Antioxidants
Cyclooxygenase 2
Diet
Dinoprostone
Fruit
Inflammation
Insurance Benefits
Interleukin-6
Macrophages
NF-kappa B
Nitric Oxide
Nitric Oxide Synthase Type II
Nutrition Surveys
Plants
Transcription Factors
Tumor Necrosis Factor-alpha
Vegetables
Antioxidants
Cyclooxygenase 2
Dinoprostone
Interleukin-6
NF-kappa B
Nitric Oxide
Nitric Oxide Synthase Type II
Transcription Factors
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Rahman I, Biswas SK, Kirkham PA. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem Pharmacol. 2006; 72:1439–1452.
Article2. Chiodoni C, Colombo MP, Sangaletti S. Matricellular proteins: from homeostasis to inflammation, cancer, and metastasis. Cancer Metastasis Rev. 2010; 29:295–307.
Article3. Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol. 2008; 8:923–934.
Article4. Mollace V, Colasanti M, Muscoli C, Lauro GM, Iannone M, Rotiroti D, Nistico G. The effect of nitric oxide on cytokine-induced release of PGE2 by human cultured astroglial cells. Br J Pharmacol. 1998; 124:742–746.
Article5. Chen CH, Sheu MT, Chen TF, Wang YC, Hou WC, Liu DZ, Chung TC, Liang YC. Suppression of endotoxin-induced proinflammatory responses by citrus pectin through blocking LPS signaling pathways. Biochem Pharmacol. 2006; 72:1001–1009.
Article6. Pison U, Max M, Neuendank A, Weissbach S, Pietschmann S. Host defence capacities of pulmonary surfactant: evidence for 'non-surfactant' functions of the surfactant system. Eur J Clin Invest. 1994; 24:586–599.
Article7. Shapira L, Soskolne WA, Houri Y, Barak V, Halabi A, Stabholz A. Protection against endotoxic shock and lipopolysaccharide-induced local inflammation by tetracycline: correlation with inhibition of cytokine secretion. Infect Immun. 1996; 64:825–828.
Article8. Zhang T, Sun L, Liu R, Zhang D, Lan X, Huang C, Xin W, Wang C, Zhang D, Du G. A novel naturally occurring salicylic acid analogue acts as an anti-inflammatory agent by inhibiting nuclear factor-kappaB activity in RAW264.7 macrophages. Mol Pharm. 2012; 9:671–677.
Article9. Verma IM. Nuclear factor (NF)-kappaB proteins: therapeutic targets. Ann Rheum Dis. 2004; 63:Suppl 2. ii57–ii61.10. Chan ED, Riches DW. IFN-gamma + LPS induction of iNOS is modulated by ERK, JNK/SAPK, and p38 (mapk) in a mouse macrophage cell line. Am J Physiol Cell Physiol. 2001; 280:C441–C450.11. Brodsky M, Halpert G, Albeck M, Sredni B. The anti-inflammatory effects of the tellurium redox modulating compound, AS101, are associated with regulation of NFkappaB signaling pathway and nitric oxide induction in macrophages. J Inflamm (Lond). 2010; 7:3.12. Pettus BJ, Bielawski J, Porcelli AM, Reames DL, Johnson KR, Morrow J, Chalfant CE, Obeid LM, Hannun YA. The sphingosine kinase 1/sphingosine-1-phosphate pathway mediates COX-2 induction and PGE2 production in response to TNF-alpha. FASEB J. 2003; 17:1411–1421.
Article13. Bacher S, Schmitz ML. The NF-kappaB pathway as a potential target for autoimmune disease therapy. Curr Pharm Des. 2004; 10:2827–2837.14. Brodsky M, Duran F, Sanhueza J, Giaccone J II. Hemodynamic study of pulmonary arterial hypertension. Rev Med Chil. 1956; 84:7–16.15. Chanput W, Mes J, Vreeburg RA, Savelkoul HF, Wichers HJ. Transcription profiles of LPS-stimulated THP-1 monocytes and macrophages: a tool to study inflammation modulating effects of food-derived compounds. Food Funct. 2010; 1:254–261.
Article16. Bravo L. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev. 1998; 56:317–333.
Article17. Di Tomo P, Canali R, Ciavardelli D, Di Silvestre S, De Marco A, Giardinelli A, Pipino C, Di Pietro N, Virgili F, Pandolfi A. beta-Carotene and lycopene affect endothelial response to TNF-alpha reducing nitro-oxidative stress and interaction with monocytes. Mol Nutr Food Res. 2012; 56:217–227.
Article18. Middleton E Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000; 52:673–751.19. Choi EM, Hwang JK. Effects of Morus alba leaf extract on the production of nitric oxide, prostaglandin E2 and cytokines in RAW264.7 macrophages. Fitoterapia. 2005; 76:608–613.
Article20. Kim SH, Oh SY. Cultural and nutritional aspects of traditional Korean diet. World Rev Nutr Diet. 1996; 79:109–132.
Article21. Kesteloot H, Zhang J. Salt consumption during the nutrition transition in South Korea. Am J Clin Nutr. 2000; 72:199–201.
Article22. Lee J, Demissie K, Lu SE, Rhoads GG. Cancer incidence among Korean-American immigrants in the United States and native Koreans in South Korea. Cancer Control. 2007; 14:78–85.
Article23. Gordon BH, Kang MS, Cho P, Sucher KP. Dietary habits and health beliefs of Korean-Americans in the San Francisco Bay Area. J Am Diet Assoc. 2000; 100:1198–1201.
Article24. Chon SU, Heo BG, Park YS, Kim DK, Gorinstein S. Total phenolics level, antioxidant activities and cytotoxicity of young sprouts of some traditional Korean salad plants. Plant Foods Hum Nutr. 2009; 64:25–31.
Article25. Kim NY, Song EJ, Kwon DY, Kim HP, Heo MY. Antioxidant and antigenotoxic activities of Korean fermented soybean. Food Chem Toxicol. 2008; 46:1184–1189.
Article26. Ministry of Health and Welfare, Korea Centers for Disease Control and Prevention. Korea Health Statistics 2010: Korea National Health and Nutrition Examination Survey (KNHANES V-1). Cheongwon: Korea Centers for Disease Control and Prevention;2011.27. Lee H, Cho MR, Chang N, Kim Y, Oh S, Kang M. Total antioxidant capacity of the Korean diet. Nutr Res Pract. 2013; Forthcoming.
Article28. Hayashi H, Tatebe S, Osaki M, Goto A, Sato K, Ito H. Anti-Fas antibody-induced apoptosis in human colorectal carcinoma cell lines: role of the p53 gene. Apoptosis. 1998; 3:431–437.29. Guesdon F, Ikebe T, Stylianou E, Warwick-Davies J, Haskill S, Saklatvala J. Interleukin 1-induced phosphorylation of MAD3, the major inhibitor of nuclear factor kappa B of HeLa cells. Interference in signalling by the proteinase inhibitors 3,4-dichloroisocoumarin and tosylphenylalanyl chloromethylketone. Biochem J. 1995; 307:287–295.
Article30. Park S, Kim J, Kim Y. Mulberry leaf extract inhibits cancer cell stemness in neuroblastoma. Nutr Cancer. 2012; 64:889–898.
Article31. Wilson KT, Ramanujam KS, Mobley HL, Musselman RF, James SP, Meltzer SJ. Helicobacter pylori stimulates inducible nitric oxide synthase expression and activity in a murine macrophage cell line. Gastroenterology. 1996; 111:1524–1533.
Article32. Tracey KJ, Cerami A. Tumor necrosis factor: a pleiotropic cytokine and therapeutic target. Annu Rev Med. 1994; 45:491–503.33. Song Y, Joung H. A traditional Korean dietary pattern and metabolic syndrome abnormalities. Nutr Metab Cardiovasc Dis. 2012; 22:456–462.
Article34. Berthet J, Damien P, Hamzeh-Cognasse H, Arthaud CA, Eyraud MA, Zéni F, Pozzetto B, McNicol A, Garraud O, Cognasse F. Human platelets can discriminate between various bacterial LPS isoforms via TLR4 signaling and differential cytokine secretion. Clin Immunol. 2012; 145:189–200.
Article35. Mihara M, Hashizume M, Yoshida H, Suzuki M, Shiina M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin Sci (Lond). 2012; 122:143–159.
Article36. Stokkers PC, Camoglio L, van Deventer SJ. Tumor necrosis factor (TNF) in inflammatory bowel disease: gene polymorphisms, animal models, and potential for anti-TNF therapy. J Inflamm. 1995; 47:97–103.37. Wang Z, Jiang W, Zhang Z, Qian M, Du B. Nitidine chloride inhibits LPS-induced inflammatory cytokines production via MAPK and NF-kappaB pathway in RAW 264.7 cells. J Ethnopharmacol. 2012; 144:145–150.
Article38. Cruz JR, Monterroso MA, Zeissig OA, Hazendonk AG, Van Wezel AL. Paralytic poliomyelitis in Guatemala. Bol Oficina Sanit Panam. 1987; 103:123–129.39. Appleby SB, Ristimäki A, Neilson K, Narko K, Hla T. Structure of the human cyclo-oxygenase-2 gene. Biochem J. 1994; 302:723–727.
Article40. Chen GY, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009; 323:1722–1725.
Article41. Deguchi Y, Kishimoto S. Tumour necrosis factor/cachectin plays a key role in autoimmune pulmonary inflammation in lupus-prone mice. Clin Exp Immunol. 1991; 85:392–395.
Article42. Aggarwal BB, Natarajan K. Tumor necrosis factors: developments during the last decade. Eur Cytokine Netw. 1996; 7:93–124.43. Harré EM, Roth J, Pehl U, Kueth M, Gerstberger R, Hübschle T. Selected contribution: role of IL-6 in LPS-induced nuclear STAT3 translocation in sensory circumventricular organs during fever in rats. J Appl Physiol (1985). 2002; 92:2657–2666.
Article44. Beg AA, Finco TS, Nantermet PV, Baldwin AS Jr. Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of I kappa B alpha: a mechanism for NF-kappa B activation. Mol Cell Biol. 1993; 13:3301–3310.
Article45. Park OJ, Surh YJ. Chemopreventive potential of epigallocatechin gallate and genistein: evidence from epidemiological and laboratory studies. Toxicol Lett. 2004; 150:43–56.
Article46. Bai SK, Lee SJ, Na HJ, Ha KS, Han JA, Lee H, Kwon YG, Chung CK, Kim YM. beta-Carotene inhibits inflammatory gene expression in lipopolysaccharide-stimulated macrophages by suppressing redox-based NF-kappaB activation. Exp Mol Med. 2005; 37:323–334.
Article47. Liu D, Shi J, Colina Ibarra A, Kakuda Y, Jun Xue S. The scavenging capacity and synergistic effects of lycopene, vitamin E, vitamin C, and β-carotene mixtures on the DPPH free radical. Lebenson Wiss Technol. 2008; 41:1344–1349.
Article48. Liu RH. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J Clin Nutr. 2003; 78:517S–520S.
Article49. Karatzi K, Papamichael C, Karatzis E, Papaioannou TG, Voidonikola PT, Vamvakou GD, Lekakis J, Zampelas A. Postprandial improvement of endothelial function by red wine and olive oil antioxidants: a synergistic effect of components of the Mediterranean diet. J Am Coll Nutr. 2008; 27:448–453.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- YJI-7 Suppresses ROS Production and Expression of Inflammatory Mediators via Modulation of p38MAPK and JNK Signaling in RAW 264.7 Macrophages
- Anti-inflammatory effects of fruit and leaf extracts of Lycium barbarum in lipopolysaccharide-stimulated RAW264.7 cells and animal model
- Anti-Inflammatory Effects of Fermented Products with Avena sativa on RAW264.7 and HT-29 Cells via Inhibition of Inflammatory Mediators
- Fermented Product Extract with Lentinus edodes Attenuate the Inflammatory Mediators Releases and Free Radical Production
- Anti-inflammatory effects of the ethanol fraction of Spiraea prunifolia var. simpliciflora in RAW 264.7 cells