Nutr Res Pract.
2014 Feb;8(1):11-19.
Ethanol extract of Synurus deltoides (Aiton) Nakai suppresses in vitro LPS-induced cytokine production in RAW 264.7 macrophages and in vivo acute inflammatory symptoms
- Affiliations
-
- 1Department of Medical Biotechnology, College of Biomedical Science, Kangwon National University, 1 Kangwondaehak-gil, Chuncheon, Gangwon 200-701, Korea. mhwang@kangwon.ac.kr
Abstract
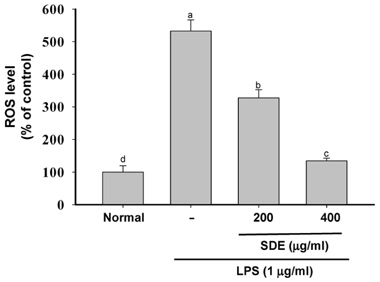
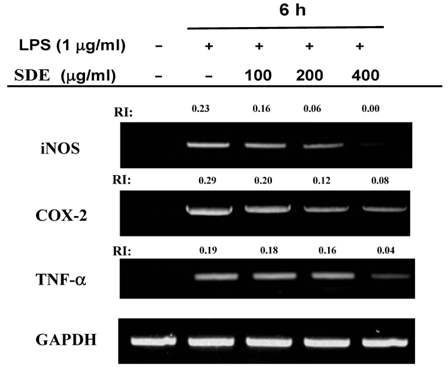
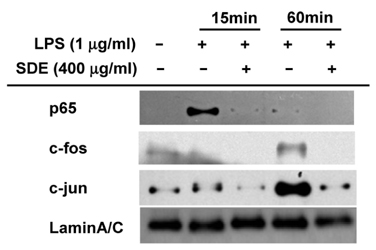
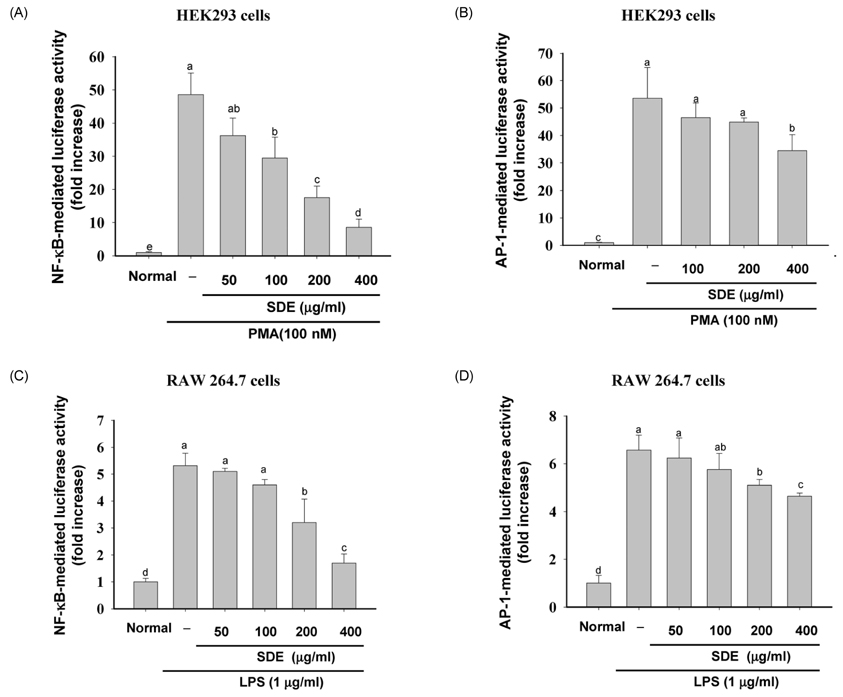
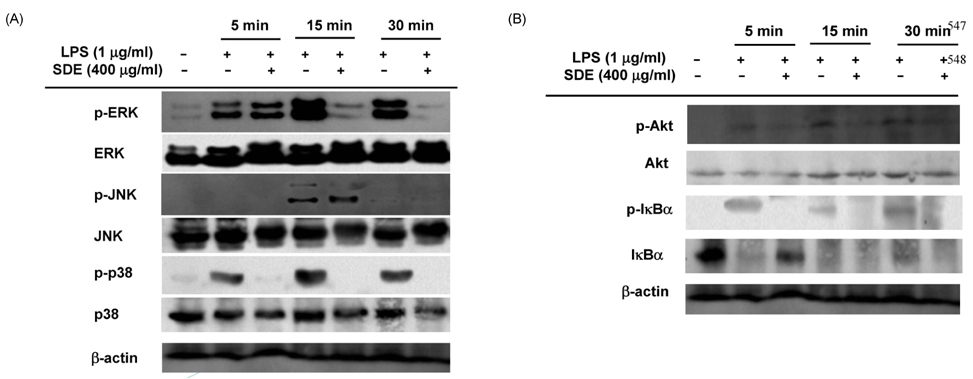
- Synurus deltoides (Aiton) Nakai, belonging to the Compositae family, is an edible plant widely distributed in Northeast Asia. In this study, we examined the mechanisms underlying the immunomodulative effects of the ethanol extract of S. deltoides (SDE). The SDE extract strongly down-regulated the mRNA expression of the inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and tumour necrosis factor (TNF)-alpha, thereby inhibiting the production of nitric oxide (NO), prostaglandin E2 (PGE2), and TNF-alpha in the lipopolysaccharide (LPS)-stimulated RAW 264.7 cells. Furthermore, SDE also suppressed the nuclear translocation of the activation protein (AP)-1 and the nuclear factor-kappaB (NF-kappaB), and simultaneously decreased the phosphorylation of extracellular signal-regulated protein kinases (ERK), p38, and Akt. In agreement with the in vitro observations, the orally administered SDE ameliorated the acute inflammatory symptoms in the arachidonic acid-induced ear edema and the EtOH/HCl-induced gastritis in mice. Therefore, S. deltoides have a potential anti-inflammatory capacity in vitro and in vivo, suggesting the potential therapeutic use in the inflammation-associated disorders.
MeSH Terms
-
Animals
Asia
Asteraceae
Cyclooxygenase 2
Dinoprostone
Ear
Edema
Ethanol*
Gastritis
Humans
Macrophages*
Mice
Necrosis
Nitric Oxide
Nitric Oxide Synthase Type II
Phosphorylation
Plants, Edible
Protein Kinases
RNA, Messenger
Tumor Necrosis Factor-alpha
Cyclooxygenase 2
Dinoprostone
Ethanol
Nitric Oxide
Nitric Oxide Synthase Type II
Protein Kinases
RNA, Messenger
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Sherwood ER, Toliver-Kinsky T. Mechanisms of the inflammatory response. Best Pract Res Clin Anaesthesiol. 2004; 18:385–405.
Article2. Lister MF, Sharkey J, Sawatzky DA, Hodgkiss JP, Davidson DJ, Rossi AG, Finlayson K. The role of the purinergic P2X7 receptor in inflammation. J Inflamm (Lond). 2007; 4:5.
Article3. Huang N, Hauck C, Yum MY, Rizshsky L, Widrlechner MP, McCoy JA, Murphy PA, Dixon PM, Nikolau BJ, Birt DF. Rosmarinic acid in Prunella vulgaris ethanol extract inhibits lipopolysaccharide-induced prostaglandin E2 and nitric oxide in RAW 264.7 mouse macrophages. J Agric Food Chem. 2009; 57:10579–10589.
Article4. Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010; 327:656–661.
Article5. Lee MS, Kim YJ. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu Rev Biochem. 2007; 76:447–480.
Article6. Lee MS, Kwon MS, Choi JW, Shin T, No HK, Choi JS, Byun DS, Kim JI, Kim HR. Anti-inflammatory activities of an ethanol extract of Ecklonia stolonifera in lipopolysaccharide-stimulated RAW 264.7 murine macrophage cells. J Agric Food Chem. 2012; 60:9120–9129.
Article7. Zhong LM, Zong Y, Sun L, Guo JZ, Zhang W, He Y, Song R, Wang WM, Xiao CJ, Lu D. Resveratrol inhibits inflammatory responses via the mammalian target of rapamycin signaling pathway in cultured LPS-stimulated microglial cells. PLoS One. 2012; 7:e32195.
Article8. Piao W, Song C, Chen H, Diaz MA, Wahl LM, Fitzgerald KA, Li L, Medvedev AE. Endotoxin tolerance dysregulates MyD88- and Toll/IL-1R domain-containing adapter inducing IFN-beta-dependent pathways and increases expression of negative regulators of TLR signaling. J Leukoc Biol. 2009; 86:863–875.
Article9. Shen T, Lee J, Lee E, Kim SH, Kim TW, Cho JY. Cafestol, a coffee-specific diterpene, is a novel extracellular signal-regulated kinase inhibitor with AP-1-targeted inhibition of prostaglandin E2 production in lipopolysaccharide-activated macrophages. Biol Pharm Bull. 2010; 33:128–132.
Article10. Ham SS, Han HS, Choi KP, Oh DH. Antigenotoxic effects of Synurus deltoides extract on benzo[a]pyrene induced mutagenesis. J Food Sci Nutr. 1997; 2:162–166.11. Choi YH, Son KH, Chang HW, Bae K, Kang SS, Kim HP. New anti-inflammatory formulation containing Synurus deltoides extract. Arch Pharm Res. 2005; 28:848–853.
Article12. Yoshitama K, Ishii K, Yasuda H. A chromatographic survey of anthocyanins in the flora of Japan, I. J Fac Sci Shinshu Univ. 1980; 15:19–26.13. Nam JH, Choi SZ, Lee KR. Phytochemical constituents of Synurus excelsus. Korean J Pharmacogn. 2004; 35:116–121.14. Lee HY, Min BS, Son KH, Chang HW, Kim HP, Kang SS, Bae KH. Cerebrosides and triterpenoids from the roots of Synurus deltoides. Nat Prod Sci. 2006; 12:193–196.15. Kang TH, Pae HO, Jeong SJ, Yoo JC, Choi BM, Jun CD, Chung HT, Miyamoto T, Higuchi R, Kim YC. Scopoletin: an inducible nitric oxide synthesis inhibitory active constituent from Artemisia feddei. Planta Med. 1999; 65:400–403.
Article16. Park JH, Son KH, Kim SW, Chang HW, Bae K, Kang SS, Kim HP. Antiinflammatory activity of Synurus deltoides. Phytother Res. 2004; 18:930–933.17. Wang J, Wang N, Yao X, Ishii R, Kitanaka S. Inhibitory activity of Chinese herbal medicines toward histamine release from mast cells and nitric oxide production by macrophage-like cell line, RAW 264.7. J Nat Med. 2006; 60:73–77.
Article18. Chon SU, Heo BG, Park YS, Cho JY, Gorinstein S. Characteristics of the leaf parts of some traditional Korean salad plants used for food. J Sci Food Agric. 2008; 88:1963–1968.
Article19. Hu W, Shen T, Wang MH. Cell cycle arrest and apoptosis induced by methyl 3,5-dicaffeoyl quinate in human colon cancer cells: involvement of the PI3K/Akt and MAP kinase pathways. Chem Biol Interact. 2011; 194:48–57.
Article20. Yu T, Ahn HM, Shen T, Yoon K, Jang HJ, Lee YJ, Yang HM, Kim JH, Kim C, Han MH, Cha SH, Kim TW, Kim SY, Lee J, Cho JY. Anti-inflammatory activity of ethanol extract derived from Phaseolus angularis beans. J Ethnopharmacol. 2011; 137:1197–1206.
Article21. Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Carter C, Yu BP, Leeuwenburgh C. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009; 8:18–30.
Article22. Wu LC, Fan NC, Lin MH, Chu IR, Huang SJ, Hu CY, Han SY. Anti-inflammatory effect of spilanthol from Spilanthes acmella on murine macrophage by down-regulating LPS-induced inflammatory mediators. J Agric Food Chem. 2008; 56:2341–2349.
Article23. Mann PB, Kennett MJ, Harvill ET. Toll-like receptor 4 is critical to innate host defense in a murine model of bordetellosis. J Infect Dis. 2004; 189:833–836.
Article24. Hu W, Wang MH. Antioxidative activity and anti-inflammatory effects of diarylheptanoids isolated from Alnus hirsuta. J Wood Sci. 2011; 57:323–330.
Article25. Hu W, Han W, Huang C, Wang MH. Protective effect of the methanolic extract from Duchesnea indica against oxidative stress in vitro and in vivo. Environ Toxicol Pharmacol. 2011; 31:42–50.
Article26. Hakim A, Adcock IM, Usmani OS. Corticosteroid resistance and novel anti-inflammatory therapies in chronic obstructive pulmonary disease: current evidence and future direction. Drugs. 2012; 72:1299–1312.
Article27. Sun J, Ramnath RD, Tamizhselvi R, Bhatia M. Role of protein kinase C and phosphoinositide 3-kinase-Akt in substance P-induced proinflammatory pathways in mouse macrophages. FASEB J. 2009; 23:997–1010.
Article28. Guha M, Mackman N. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J Biol Chem. 2002; 277:32124–32132.
Article29. Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007; 19:142–149.
Article30. Adcock IM, Caramori G. Cross-talk between pro-inflammatory transcription factors and glucocorticoids. Immunol Cell Biol. 2001; 79:376–384.
Article31. Byeon SE, Chung JY, Lee YG, Kim BH, Kim KH, Cho JY. In vitro and in vivo anti-inflammatory effects of taheebo, a water extract from the inner bark of Tabebuia avellanedae. J Ethnopharmacol. 2008; 119:145–152.
Article32. Yang Y, Lee GJ, Yoon DH, Yu T, Oh J, Jeong D, Lee J, Kim SH, Kim TW, Cho JY. ERK1- and TBK1-targeted anti-inflammatory activity of an ethanol extract of Dryopteris crassirhizoma. J Ethnopharmacol. 2013; 145:499–508.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Coumarins from Roots of Paramignya scandens (Griff.) Craib on LPS-induced IL-1β and IL-10 Cytokine Production in RAW 264.7 Macrophages
- The Anti-Inflammatory Effect of Pegmatite by in Vivo and in Vitro Study
- Anti-inflammatory Effect of Mangosteen (Garcinia mangostana L.) Peel Extract and its Compounds in LPS-induced RAW264.7 Cells
- Anti-Inflammatory Effects of Chrysanthemum indicum Water Extract in RAW 264.7 Cell as a Whole Plant
- Anti-inflammatory effects of ethanolic extract of Annona muricata