Nutr Res Pract.
2013 Dec;7(6):423-429.
Luteolin and luteolin-7-O-glucoside inhibit lipopolysaccharide-induced inflammatory responses through modulation of NF-kappaB/AP-1/PI3K-Akt signaling cascades in RAW 264.7 cells
- Affiliations
-
- 1Department of Clinical Laboratory Science, Dong-Eui University, Busan 614-714, Korea.
- 2Department of Smart Foods and Drugs, Inje University, 607 Obang-dong, Gimhae, Gyeongnam 621-749, Korea. fdsnsong@inje.ac.kr
Abstract
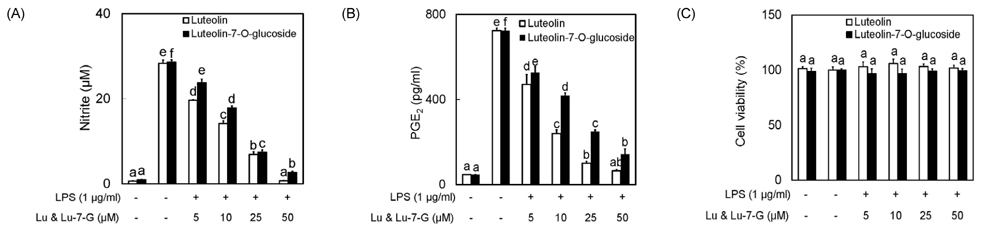
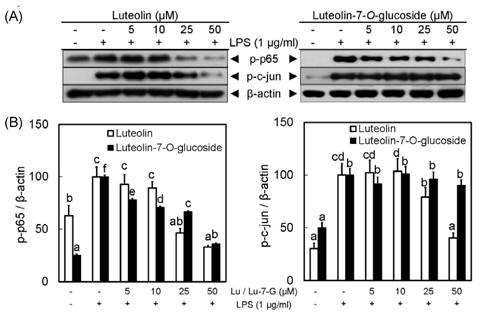
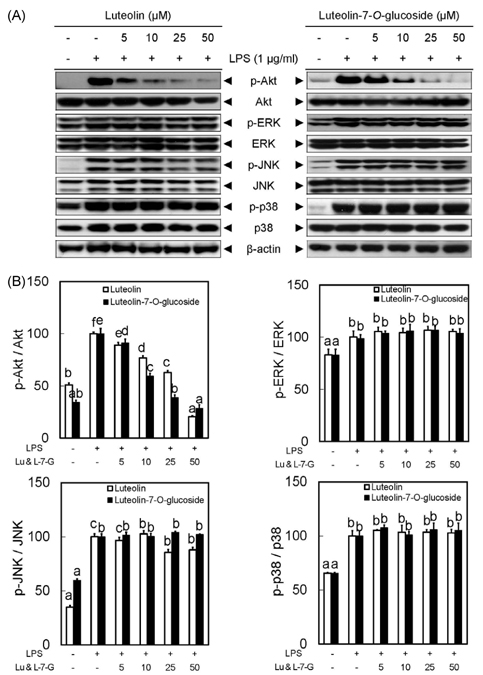
- Luteolin is a flavonoid found in abundance in celery, green pepper, and dandelions. Previous studies have shown that luteolin is an anti-inflammatory and anti-oxidative agent. In this study, the anti-inflammatory capacity of luteolin and one of its glycosidic forms, luteolin-7-O-glucoside, were compared and their molecular mechanisms of action were analyzed. In lipopolysaccharide (LPS)-activated RAW 264.7 cells, luteolin more potently inhibited the production of nitric oxide (NO) and prostaglandin E2 as well as the expression of their corresponding enzymes (inducible NO synthase (iNOS) and cyclooxygenase-2 (COX-2) than luteolin-7-O-glucoside. The molecular mechanisms underlying these effects were investigated to determine whether the inflammatory response was related to the transcription factors, nuclear factor (NF)-kappaB and activator protein (AP)-1, or their upstream signaling molecules, mitogen-activated protein kinases (MAPKs) and phosphoinositide 3-kinase (PI3K). Luteolin attenuated the activation of both transcription factors, NF-kappaB and AP-1, while luteolin-7-O-glucoside only impeded NF-kappaB activation. However, both flavonoids inhibited Akt phosphorylation in a dose-dependent manner. Consequently, luteolin more potently ameliorated LPS-induced inflammation than luteolin-7-O-glucoside, which might be attributed to the differentially activated NF-kappaB/AP-1/PI3K-Akt pathway in RAW 264.7 cells.
MeSH Terms
-
Apium graveolens
Capsicum
Cyclooxygenase 2
Dinoprostone
Flavones
Flavonoids
Glucosides
Inflammation
Luteolin*
Mitogen-Activated Protein Kinases
NF-kappa B
Nitric Oxide
Nitric Oxide Synthase
Phosphorylation
Taraxacum
Transcription Factor AP-1
Transcription Factors
Cyclooxygenase 2
Dinoprostone
Flavones
Flavonoids
Glucosides
Luteolin
Mitogen-Activated Protein Kinases
NF-kappa B
Nitric Oxide
Nitric Oxide Synthase
Transcription Factor AP-1
Transcription Factors
Figure
Reference
-
1. Surh YJ, Chun KS, Cha HH, Han SS, Keum YS, Park KK, Lee SS. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat Res. 2001; 480-481:243–268.
Article2. Fujioka S, Niu J, Schmidt C, Sclabas GM, Peng B, Uwagawa T, Li Z, Evans DB, Abbruzzese JL, Chiao PJ. NF-kappaB and AP-1 connection: mechanism of NF-kappaB-dependent regulation of AP-1 activity. Mol Cell Biol. 2004; 24:7806–7819.
Article3. Kim YW, West XZ, Byzova TV. Inflammation and oxidative stress in angiogenesis and vascular disease. J Mol Med (Berl). 2013; 91:323–328.
Article4. Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003; 3:768–780.
Article5. Biesalski HK. Polyphenols and inflammation: basic interactions. Curr Opin Clin Nutr Metab Care. 2007; 10:724–728.
Article6. Middleton E Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000; 52:673–751.7. Richelle M, Pridmore-Merten S, Bodenstab S, Enslen M, Offord EA. Hydrolysis of isoflavone glycosides to aglycones by β-glycosidase does not alter plasma and urine isoflavone pharmacokinetics in postmenopausal women. J Nutr. 2002; 132:2587–2592.
Article8. Murota K, Shimizu S, Miyamoto S, Izumi T, Obata A, Kikuchi M, Terao J. Unique uptake and transport of isoflavone aglycones by human intestinal Caco-2 cells: comparison of isoflavonoids and flavonoids. J Nutr. 2002; 132:1956–1961.
Article9. Zhou P, Li LP, Luo SQ, Jiang HD, Zeng S. Intestinal absorption of luteolin from peanut hull extract is more efficient than that from individual pure luteolin. J Agric Food Chem. 2008; 56:296–300.
Article10. Izumi T, Piskula MK, Osawa S, Obata A, Tobe K, Saito M, Kataoka S, Kubota Y, Kikuchi M. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J Nutr. 2000; 130:1695–1699.
Article11. Kano M, Takayanagi T, Harada K, Sawada S, Ishikawa F. Bioavailability of isoflavones after ingestion of soy beverages in healthy adults. J Nutr. 2006; 136:2291–2296.
Article12. Andlauer W, Kolb J, Fürst P. Isoflavones from tofu are absorbed and metabolized in the isolated rat small intestine. J Nutr. 2000; 130:3021–3027.
Article13. Piskula MK. Factors affecting flavonoids absorption. Biofactors. 2000; 12:175–180.
Article14. Zubik L, Meydani M. Bioavailability of soybean isoflavones from aglycone and glucoside forms in American women. Am J Clin Nutr. 2003; 77:1459–1465.
Article15. Seelinger G, Merfort I, Schempp CM. Anti-oxidant, anti-inflammatory and anti-allergic activities of luteolin. Planta Med. 2008; 74:1667–1677.
Article16. Rowland I, Faughnan M, Hoey L, Wähälä K, Williamson G, Cassidy A. Bioavailability of phyto-oestrogens. Br J Nutr. 2003; 89:Suppl 1. S45–S58.
Article17. Chen CY, Peng WH, Tsai KD, Hsu SL. Luteolin suppresses inflammation-associated gene expression by blocking NF-kappaB and AP-1 activation pathway in mouse alveolar macrophages. Life Sci. 2007; 81:1602–1614.
Article18. Hu C, Kitts DD. Luteolin and luteolin-7-O-glucoside from dandelion flower suppress iNOS and COX-2 in RAW264.7 cells. Mol Cell Biochem. 2004; 265:107–113.
Article19. Hwang JT, Park OJ, Lee YK, Sung MJ, Hur HJ, Kim MS, Ha JH, Kwon DY. Anti-tumor effect of luteolin is accompanied by AMP-activated protein kinase and nuclear factor-κB modulation in HepG2 hepatocarcinoma cells. Int J Mol Med. 2011; 28:25–31.
Article20. Park CM, Park JY, Noh KH, Shin JH, Song YS. Taraxacum officinale Weber extracts inhibit LPS-induced oxidative stress and nitric oxide production via the NF-κB modulation in RAW 264.7 cells. J Ethnopharmacol. 2011; 133:834–842.
Article21. Park CM, Jin KS, Lee YW, Song YS. Luteolin and chicoric acid synergistically inhibited inflammatory responses via inactivation of PI3K-Akt pathway and impairment of NF-κB translocation in LPS stimulated RAW 264.7 cells. Eur J Pharmacol. 2011; 660:454–459.
Article22. Schütz K, Kammerer DR, Carle R, Schieber A. Characterization of phenolic acids and flavonoids in dandelion (Taraxacum officinale WEB. ex WIGG.) root and herb by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom. 2005; 19:179–186.
Article23. Jin M, Yang JH, Lee E, Lu Y, Kwon S, Son KH, Son JK, Chang HW. Antiasthmatic activity of luteolin-7-O-glucoside from Ailanthus altissima through the downregulation of T helper 2 cytokine expression and inhibition of prostaglandin E2 production in an ovalbumin-induced asthma model. Biol Pharm Bull. 2009; 32:1500–1503.
Article24. Verschooten L, Smaers K, Van Kelst S, Proby C, Maes D, Declercq L, Agostinis P, Garmyn M. The flavonoid luteolin increases the resistance of normal, but not malignant keratinocytes, against UVB-induced apoptosis. J Invest Dermatol. 2010; 130:2277–2285.
Article25. Baskar AA, Ignacimuthu S, Michael GP, Al Numair KS. Cancer chemopreventive potential of luteolin-7-O-glucoside isolated from Ophiorrhiza mungos Linn. Nutr Cancer. 2011; 63:130–138.26. Jung HA, Jin SE, Min BS, Kim BW, Choi JS. Anti-inflammatory activity of Korean thistle Cirsium maackii and its major flavonoid, luteolin 5-O-glucoside. Food Chem Toxicol. 2012; 50:2171–2179.
Article27. Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002; 22:19–34.
Article28. Hollman PC, Katan MB. Health effects and bioavailability of dietary flavonols. Free Radic Res. 1999; 31:Suppl. S75–S80.
Article29. Lee JP, Li YC, Chen HY, Lin RH, Huang SS, Chen HL, Kuan PC, Liao MF, Chen CJ, Kuan YH. Protective effects of luteolin against lipopolysaccharide-induced acute lung injury involves inhibition of MEK/ERK and PI3K/Akt pathways in neutrophils. Acta Pharmacol Sin. 2010; 31:831–838.
Article30. Jang S, Kelley KW, Johnson RW. Luteolin reduces IL-6 production in microglia by inhibiting JNK phosphorylation and activation of AP-1. Proc Natl Acad Sci U S A. 2008; 105:7534–7539.
Article31. Park CM, Jin KS, Cho CW, Lee YW, Huh GH, Cha YS, Song YS. Luteolin inhibits inflammatory responses by down-regulating the JNK-NFκB and AP-1 pathways in TNF-α activated HepG2 cells. Food Sci Biotechnol. 2012; 21:279–283.
Article32. Oh J, Kim JH, Park JG, Yi YS, Park KW, Rho HS, Lee MS, Yoo JW, Kang SH, Hong YD, Shin SS, Cho JY. Radical scavenging activity-based and AP-1-targeted anti-inflammatory effects of lutein in macrophage-like and skin keratinocytic cells. Mediators Inflamm. 2013; 2013:787042.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Luteolin 5-O-glucoside from Korean Milk Thistle, Cirsium maackii, Exhibits Anti-Inflammatory Activity via Activation of the Nrf2/HO-1 Pathway
- Luteolin and luteolin-7-O-glucoside protect against acute liver injury through regulation of inflammatory mediators and antioxidative enzymes in GalN/LPS-induced hepatitic ICR mice
- Methyl p-Hydroxycinnamate Suppresses Lipopolysaccharide-Induced Inflammatory Responses through Akt Phosphorylation in RAW264.7 Cells
- Aromadendrin Inhibits Lipopolysaccharide-Induced Nuclear Translocation of NF-kappaB and Phosphorylation of JNK in RAW 264.7 Macrophage Cells
- Phosphorylation of Akt Mediates Anti-Inflammatory Activity of 1-p-Coumaroyl beta-D-Glucoside Against Lipopolysaccharide-Induced Inflammation in RAW264.7 Cells