Nat Prod Sci.
2017 Sep;23(3):183-191. 10.20307/nps.2017.23.3.183.
Luteolin 5-O-glucoside from Korean Milk Thistle, Cirsium maackii, Exhibits Anti-Inflammatory Activity via Activation of the Nrf2/HO-1 Pathway
- Affiliations
-
- 1Department of Food Science and Human Nutrition, Chonbuk National University, Jeonju 54896, Republic of Korea.
- 2Department of Food and Life Science, Pukyong National University, Busan 48513, Republic of Korea. choijs@pknu.ac.kr
- 3Department of Pharmaceutical Engineering, Sangji University, Wonju 220-702, Republic of Korea.
- KMID: 2393799
- DOI: http://doi.org/10.20307/nps.2017.23.3.183
Abstract
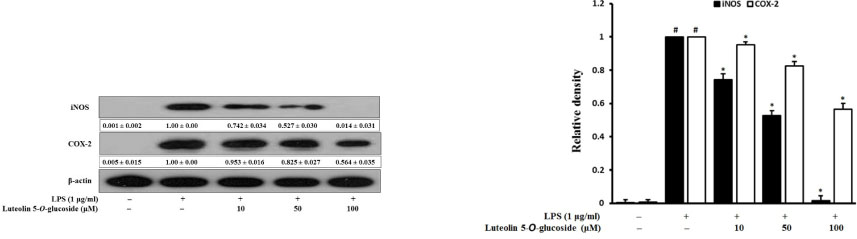
- Luteolin 5-O-glucoside is the major flavonoid from Korean thistle, Cirsium maackii. We previously reported the anti-inflammatory activities of luteolin 5-O-glucoside in lipopolysaccharide (LPS)-stimulated RAW 264.7 cells. In this study, we determined the anti-inflammatory mechanisms of luteolin 5-O-glucoside through the inhibition of nitric oxide (NO) production in vitro and in vivo. Results revealed that luteolin 5-O-glucoside dose-dependently inhibited NO production and expression of iNOS and COX-2 in LPS-induced RAW 264.7 cells. Luteolin 5-O-glucoside also significantly inhibited the translocation of NF-κB, the activation of MAPKs, and ROS generation in LPS-induced RAW 264.7 cells. In addition, protein expressions of Nrf-2 and HO-1 were also upregulated by luteolin 5-O-glucoside treatment. Moreover, luteolin 5-O-glucoside inhibited λ-carrageenan-induced mouse paw edema by 65.34% and 48.31% at doses of 50 and 100 mg/kg body weight, respectively. These findings indicate potential anti-inflammatory effect of luteolin 5-O-glucoside particularly by downregulating NF-κB and upregulating HO-1/Nrf-2 pathway.
Keyword
MeSH Terms
Figure
Reference
-
1. Choudhari AS, Raina P, Deshpande MM, Wali AG, Zanwar A, Bodhankar SL, Kaul-Ghanekar R. J Ethnopharmacol. 2013; 150:215–222.2. Joung EJ, Lee B, Gwon WG, Shin T, Jung BM, Yoon NY, Choi JS, Oh CW, Kim HR. Int Immunopharmacol. 2015; 29:693–700.3. Giuliani C, Napolitano G, Bucci I, Montani V, Monaco F. Clin Ter. 2001; 152:249–253.4. May MJ, Ghosh S. Immunol Today. 1998; 19:80–88.5. Tak PP, Firestein GS. J Clin Invest. 2001; 107:7–11.6. Kim AR, Lee MS, Shin TS, Hua H, Jang BC, Choi JS, Byun DS, Utsuki T, Ingram D, Kim HR. Toxicol In Vitro. 2011; 25:1789–1795.7. Pae HO, Chung HT. Immune Netw. 2009; 9:12–19.8. Chen TY, Sun HL, Yao HT, Lii CK, Chen HW, Chen PY, Li CC, Liu KL. Food Chem Toxicol. 2013; 55:257–264.9. Taha R, Blaise G. Funct Food Health Dis. 2014; 4:510–523.10. Kim JG. Illustrated Natural Drugs Encyclopedia. Korea: Namsandang;1984. p. 37.11. Iwashina T, Ito T, Ootani S. Ann Tsukuba Bot Gard. 1989; 8:15–19.12. Jung HA, Kim YS, Choi JS. Food Chem Toxicol. 2009; 47:2790–2797.13. Jung HA, Jin SE, Min BS, Kim BW, Choi JS. Food Chem Toxicol. 2012; 50:2171–2179.14. Morris CJ. Methods Mol Biol. 2003; 225:115–121.15. Sharif O, Bolshakov VN, Raines S, Newham P, Perkins ND. BMC Immunol. 2007; 8:1–17.
Article16. Lee CB. Flora of Korea. Korea: Hyangmoonsa;1979. p. 274.17. Lee SJ. Korean Folk Medicine. Korea: Seoul National University;1966. p. 145–146.18. Keiser K, Johnson CC, Tipton DA. J Endod. 2000; 26:288–291.19. Meda L, Cassatella MA, Szendrei GI, Otvos L Jr, Baron P, Villalba M, Ferrari D, Rossi F. Nature. 1995; 374:647–650.20. Dandona P, Chaudhuri A, Dhindsa S. Diabetes Care. 2010; 33:1686–1687.21. Marks-Konczalik J, Chu SC, Moss J. J Biol Chem. 1998; 273:22201–22208.22. Islam MN, Choi RJ, Jin SE, Kim YS, Ahn BR, Zhao D, Jung HA, Choi JS. J Ethnopharmacol. 2012; 144:175–181.23. Chen JJ, Huang WC, Chen CC. Mol Biol Cell. 2005; 16:5579–5591.24. Rajapakse N, Kim MM, Mendis E, Kim SK. Immunology. 2008; 123:348–357.25. Kaminska B. Biochim Biophys Acta. 2005; 1754:253–262.26. Hancock JT, Desikan R, Neill SJ. Biochem Soc Trans. 2001; 29:345–350.27. Choi SY, Hwang JH, Ko HC, Park JG, Kim SJ. J Ethnopharmacol. 2007; 113:149–155.28. Siomek A. Acta Biochim Pol. 2012; 59:323–331.29. Ryan KA, Smith MF Jr, Sanders MK, Ernst PB. Infect Immun. 2004; 72:2123–2130.30. Kim JH, Choo YY, Tae N, Min BS, Lee JH. Int Immunopharmacol. 2014; 22:420–426.31. Lee IS, Lim J, Gal J, Kang JC, Kim HJ, Kang BY, Choi HJ. Neurochem Int. 2011; 58:153–160.32. Paine A, Eiz-Vesper B, Blasczyk R, Immenschuh S. Biochem Pharmacol. 2010; 80:1895–1903.33. Lee MY, Lee JA, Seo CS, Ha H, Lee H, Son JK, Shin HK. Food Chem Toxicol. 2011; 49:1047–1055.34. Tsoyi K, Lee TY, Lee YS, Kim HJ, Seo HG, Lee JH, Chang KC. Mol Pharmacol. 2009; 76:173–182.35. Salvemini D, Wang ZQ, Bourdon DM, Stern MK, Currie MG, Manning PT. Eur J Pharmacol. 1996; 303:217–220.36. Rocha AC, Fernandes ES, Quintão NL, Campos MM, Calixto JB. Br J Pharmacol. 2006; 148:688–695.37. Yuan G, Wahlqvist ML, He G, Yang M, Li D. Asia Pac J Clin Nutr. 2006; 15:143–152.38. Camuesco D, Comalada M, Rodríguez-Cabezas ME, Nieto A, Lorente MD, Concha A, Zarzuelo A, Gálvez J. Br J Pharmacol. 2004; 143:908–918.39. Halliwell B, Zhao K, Whiteman M. Free Radic Res. 2000; 33:819–830.40. Comalada M, Ballester I, Bailón E, Sierra S, Xaus J, Gálvez J, de Medina FS, Zarzuelo A. Biochem Pharmacol. 2006; 72:1010–1021.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Isolation and Quantitative Analysis of BACE1 Inhibitory Compounds from Cirsium maackii Flower
- Luteolin and luteolin-7-O-glucoside inhibit lipopolysaccharide-induced inflammatory responses through modulation of NF-kappaB/AP-1/PI3K-Akt signaling cascades in RAW 264.7 cells
- Luteolin and luteolin-7-O-glucoside protect against acute liver injury through regulation of inflammatory mediators and antioxidative enzymes in GalN/LPS-induced hepatitic ICR mice
- Luteolin Promotes Apoptosis of Endometriotic Cells and Inhibits the Alternative Activation of Endometriosis-Associated Macrophages
- Fraxetin Induces Heme Oxygenase-1 Expression by Activation of Akt/Nrf2 or AMP-activated Protein Kinase α/Nrf2 Pathway in HaCaT Cells