Nutr Res Pract.
2019 Dec;13(6):473-479. 10.4162/nrp.2019.13.6.473.
Luteolin and luteolin-7-O-glucoside protect against acute liver injury through regulation of inflammatory mediators and antioxidative enzymes in GalN/LPS-induced hepatitic ICR mice
- Affiliations
-
- 1Department of Clinical Laboratory Science, Dong-Eui University, Busan 47340, Korea.
- 2Department of Smart Foods and Drugs, Inje University, 197 Inje-ro, Gimhae, Gyeongnam 50834, Korea. fdsnsong@inje.ac.kr
- KMID: 2464126
- DOI: http://doi.org/10.4162/nrp.2019.13.6.473
Abstract
- BACKGROUND/OBJECTIVES
Anti-inflammatory and antioxidative activities of luteolin and luteolin-7-O-glucoside were compared in galactosamine (GalN)/lipopolysaccharide (LPS)-induced hepatitic ICR mice.
MATERIALS/METHODS
Male ICR mice (6 weeks old) were divided into 4 groups: normal control, GalN/LPS, luteolin, and luteolin-7-O-glucoside groups. The latter two groups were administered luteolin or luteolin-7-O-glucoside (50 mg/kg BW) daily by gavage for 3 weeks after which hepatitis was induced by intraperitoneal injection of GalN and LPS (1 g/kg BW and 10 µg/kg BW, respectively).
RESULTS
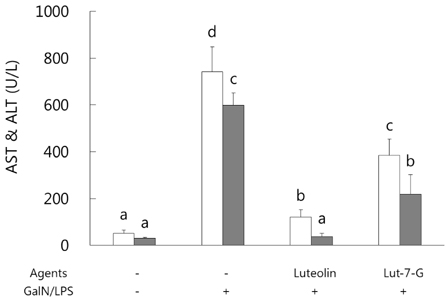
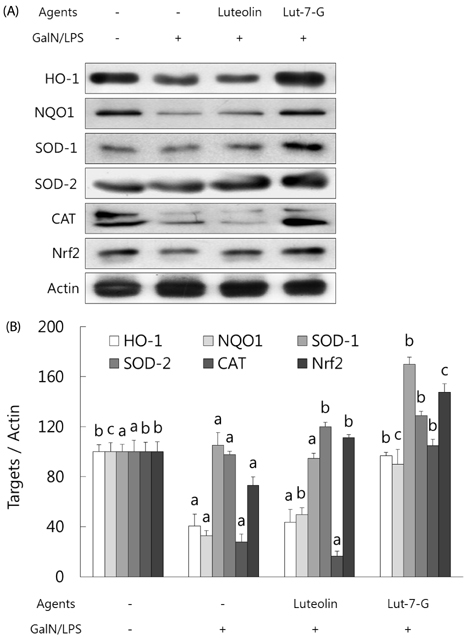
GalN/LPS produced acute hepatic injury by a sharp increase in serum AST, ALT, and TNF-α levels, increases that were ameliorated in the experimental groups. In addition, markedly increased expressions of cyclooxygenase (COX)-2 and its transcription factors, nuclear factor (NF)-κB and activator protein (AP)-1, were also significantly attenuated in the experimental groups. Compared to luteolin-7-O-glucoside, luteolin more potently ameliorated the levels of inflammatory mediators. Phase II enzymes levels and NF-E2 p45-related factor (Nrf)-2 activation that were decreased by GalN/LPS were increased by luteolin and luteolin-7-O-glucoside administration. In addition, compared to luteolin, luteolin-7-O-glucoside acted as a more potent inducer of changes in phase II enzymes. Liver histopathology results were consistent with the mediator and enzyme results.
CONCLUSION
Luteolin and luteolin-7-O-glucoside protect against GalN/LPS-induced hepatotoxicity through the regulation of inflammatory mediators and phase II enzymes.
MeSH Terms
-
Animals
Galactosamine
Hepatitis
Humans
Inflammation
Injections, Intraperitoneal
Liver*
Luteolin*
Male
Mice
Mice, Inbred ICR*
NF-E2-Related Factor 2
NF-kappa B
Prostaglandin-Endoperoxide Synthases
Transcription Factors
Galactosamine
Luteolin
NF-E2-Related Factor 2
NF-kappa B
Prostaglandin-Endoperoxide Synthases
Transcription Factors
Figure
Cited by 1 articles
-
L -Methionine inhibits 4-hydroxy-2-nonenal accumulation and suppresses inflammation in growing rats
Zhengxuan Wang, Mingcai Liang, Hui Li, Bingxiao Liu, Lin Yang
Nutr Res Pract. 2022;16(6):729-744. doi: 10.4162/nrp.2022.16.6.729.
Reference
-
1. Fei J, Liang B, Jiang C, Ni H, Wang L. Luteolin inhibits IL-1β-induced inflammation in rat chondrocytes and attenuates osteoarthritis progression in a rat model. Biomed Pharmacother. 2019; 109:1586–1592.
Article2. Seelinger G, Merfort I, Schempp CM. Anti-oxidant, anti-inflammatory and anti-allergic activities of luteolin. Planta Med. 2008; 74:1667–1677.
Article3. Richelle M, Pridmore-Merten S, Bodenstab S, Enslen M, Offord EA. Hydrolysis of isoflavone glycosides to aglycones by beta-glycosidase does not alter plasma and urine isoflavone pharmacokinetics in postmenopausal women. J Nutr. 2002; 132:2587–2592.
Article4. Izumi T, Piskula MK, Osawa S, Obata A, Tobe K, Saito M, Kataoka S, Kubota Y, Kikuchi M. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J Nutr. 2000; 130:1695–1699.
Article5. Kano M, Takayanagi T, Harada K, Sawada S, Ishikawa F. Bioavailability of isoflavones after ingestion of soy beverages in healthy adults. J Nutr. 2006; 136:2291–2296.
Article6. Andlauer W, Kolb J, Fürst P. Isoflavones from tofu are absorbed and metabolized in the isolated rat small intestine. J Nutr. 2000; 130:3021–3027.
Article7. Rowland I, Faughnan M, Hoey L, Wähälä K, Williamson G, Cassidy A. Bioavailability of phyto-oestrogens. Br J Nutr. 2003; 89 Suppl 1:S45–S58.
Article8. Kure A, Nakagawa K, Kondo M, Kato S, Kimura F, Watanabe A, Shoji N, Hatanaka S, Tsushida T, Miyazawa T. Metabolic fate of luteolin in rats: its relationship to anti-inflammatory effect. J Agric Food Chem. 2016; 64:4246–4254.
Article9. Zhou P, Li LP, Luo SQ, Jiang HD, Zeng S. Intestinal absorption of luteolin from peanut hull extract is more efficient than that from individual pure luteolin. J Agric Food Chem. 2008; 56:296–300.
Article10. Piskula MK. Factors affecting flavonoids absorption. Biofactors. 2000; 12:175–180.
Article11. Zubik L, Meydani M. Bioavailability of soybean isoflavones from aglycone and glucoside forms in American women. Am J Clin Nutr. 2003; 77:1459–1465.
Article12. Kemelo MK, Wojnarová L, Kutinová Canová N, Farghali H. D-galactosamine/lipopolysaccharide-induced hepatotoxicity downregulates sirtuin 1 in rat liver: role of sirtuin 1 modulation in hepatoprotection. Physiol Res. 2014; 63:615–623.
Article13. Ferencíková R, Cervinková Z, Drahota Z. Hepatotoxic effect of D-galactosamine and protective role of lipid emulsion. Physiol Res. 2003; 52:73–78.14. Surh YJ, Chun KS, Cha HH, Han SS, Keum YS, Park KK, Lee SS. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat Res. 2001; 480-481:243–268.
Article15. Fujioka S, Niu J, Schmidt C, Sclabas GM, Peng B, Uwagawa T, Li Z, Evans DB, Abbruzzese JL, Chiao PJ. NF-kappaB and AP-1 connection: mechanism of NF-kappaB-dependent regulation of AP-1 activity. Mol Cell Biol. 2004; 24:7806–7819.
Article16. Sajadimajd S, Khazaei M. Oxidative stress and cancer: the role of Nrf2. Curr Cancer Drug Targets. 2018; 18:538–557.
Article17. Zhang M, An C, Gao Y, Leak RK, Chen J, Zhang F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog Neurobiol. 2013; 100:30–47.
Article18. Mao J, Yi M, Wang R, Huang Y, Chen M. Protective effects of costunolide against D-galactosamine and lipopolysaccharide-induced acute liver injury in mice. Front Pharmacol. 2018; 9:1469.
Article19. Aziz N, Kim MY, Cho JY. Anti-inflammatory effects of luteolin: a review of in vitro, in vivo, and in silico studies. J Ethnopharmacol. 2018; 225:342–358.
Article20. Jia Z, Nallasamy P, Liu D, Shah H, Li JZ, Chitrakar R, Si H, McCormick J, Zhu H, Zhen W, Li Y. Luteolin protects against vascular inflammation in mice and TNF-alpha-induced monocyte adhesion to endothelial cells via suppressing IΚBα/NF-κB signaling pathway. J Nutr Biochem. 2015; 26:293–302.
Article21. Park CM, Song YS. Luteolin and luteolin-7-O-glucoside inhibit lipopolysaccharide-induced inflammatory responses through modulation of NF-κB/AP-1/PI3K-Akt signaling cascades in RAW 264.7 cells. Nutr Res Pract. 2013; 7:423–429.
Article22. Song YS, Park CM. Luteolin and luteolin-7-O-glucoside strengthen antioxidative potential through the modulation of Nrf2/MAPK mediated HO-1 signaling cascade in RAW 264.7 cells. Food Chem Toxicol. 2014; 65:70–75.
Article23. Lee WC, Jung HA, Choi JS, Kim YS, Lee SM. Protective effects of luteolin against apoptotic liver damage induced by D-galactosamine/lipopolysaccharide in mice. J Nat Prod. 2011; 74:1916–1921.
Article24. Hossen MJ, Yang WS, Kim D, Aravinthan A, Kim JH, Cho JY. Thymoquinone: an IRAK1 inhibitor with in vivo and in vitro anti-inflammatory activities. Sci Rep. 2017; 7:42995.
Article25. Wu YH, Hu SQ, Liu J, Cao HC, Xu W, Li YJ, Li LJ. Nature and mechanisms of hepatocyte apoptosis induced by D-galactosamine/lipopolysaccharide challenge in mice. Int J Mol Med. 2014; 33:1498–1506.
Article26. Shanmugam S, Thangaraj P, Lima BD, Chandran R, de Souza Araújo AA, Narain N, Serafini MR, Júnior LJ. Effects of luteolin and quercetin 3-β-d-glucoside identified from Passiflora subpeltata leaves against acetaminophen induced hepatotoxicity in rats. Biomed Pharmacother. 2016; 83:1278–1285.
Article27. Jiang W, Sun R, Wei H, Tian Z. Toll-like receptor 3 ligand attenuates LPS-induced liver injury by down-regulation of toll-like receptor 4 expression on macrophages. Proc Natl Acad Sci U S A. 2005; 102:17077–17082.
Article28. Nowak M, Gaines GC, Rosenberg J, Minter R, Bahjat FR, Rectenwald J, MacKay SL, Edwards CK 3rd, Moldawer LL. LPS-induced liver injury in D-galactosamine-sensitized mice requires secreted TNF-α and the TNF-p55 receptor. Am J Physiol Regul Integr Comp Physiol. 2000; 278:R1202–R1209.29. Hossen MJ, Kim MY, Kim JH, Cho JY. AP-1-targeted inhibition of macrophage function and lipopolysaccharide/D-galactosamine-induced hepatitis by Phyllanthus acidus methanolic extract. Am J Chin Med. 2015; 43:1137–1158.
Article30. Dejager L, Libert C. Tumor necrosis factor alpha mediates the lethal hepatotoxic effects of poly(I:C) in D-galactosamine-sensitized mice. Cytokine. 2008; 42:55–61.
Article31. Kim YW, West XZ, Byzova TV. Inflammation and oxidative stress in angiogenesis and vascular disease. J Mol Med (Berl). 2013; 91:323–328.
Article32. Lee JP, Li YC, Chen HY, Lin RH, Huang SS, Chen HL, Kuan PC, Liao MF, Chen CJ, Kuan YH. Protective effects of luteolin against lipopolysaccharide-induced acute lung injury involves inhibition of MEK/ERK and PI3K/Akt pathways in neutrophils. Acta Pharmacol Sin. 2010; 31:831–838.
Article33. Park CM, Jin KS, Cho CW, Lee YW, Huh GH, Cha YS, Song YS. Luteolin inhibits inflammatory responses by down-regulating the JNK- NFκB and AP-1 pathways in TNF-α activated HepG2 cells. Food Sci Biotechnol. 2012; 21:279–283.
Article34. Shimoi K, Okada H, Furugori M, Goda T, Takase S, Suzuki M, Hara Y, Yamamoto H, Kinae N. Intestinal absorption of luteolin and luteolin 7-O-beta-glucoside in rats and humans. FEBS Lett. 1998; 438:220–224.
Article35. Murota K, Shimizu S, Miyamoto S, Izumi T, Obata A, Kikuchi M, Terao J. Unique uptake and transport of isoflavone aglycones by human intestinal caco-2 cells: comparison of isoflavonoids and flavonoids. J Nutr. 2002; 132:1956–1961.
Article36. Hollman PC, Katan MB. Health effects and bioavailability of dietary flavonols. Free Radic Res. 1999; 31 Suppl:S75–S80.
Article37. Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002; 22:19–34.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Luteolin 5-O-glucoside from Korean Milk Thistle, Cirsium maackii, Exhibits Anti-Inflammatory Activity via Activation of the Nrf2/HO-1 Pathway
- Luteolin and luteolin-7-O-glucoside inhibit lipopolysaccharide-induced inflammatory responses through modulation of NF-kappaB/AP-1/PI3K-Akt signaling cascades in RAW 264.7 cells
- Luteolin Protects Cardiomyocytes Cells against Lipopolysaccharide-Induced Apoptosis and Inflammatory Damage by Modulating Nlrp3
- Inhibitory Effect of Luteolin Liposome Solution by Animal Model for Atopic Dermatitis in NC/Nga Mice
- Luteolin Induces the Differentiation of Osteoblasts