Nutr Res Pract.
2013 Jun;7(3):185-191.
Effect of dietary legumes on bone-specific gene expression in ovariectomized rats
- Affiliations
-
- 1Department of Food and Nutrition, Hanyang University, 222 Wangsimni-ro, Seongdong-gu, Seoul 133-791, Korea. yongsoon@hanyang.ac.kr
- 2Department of Anatomy and Cell Biology, College of Medicine, Hanyang University, Seoul 133-791, Korea.
- 3Department of Nuclear Medicine, College of Medicine, Kyung Hee University, Seoul 130-702, Korea.
Abstract
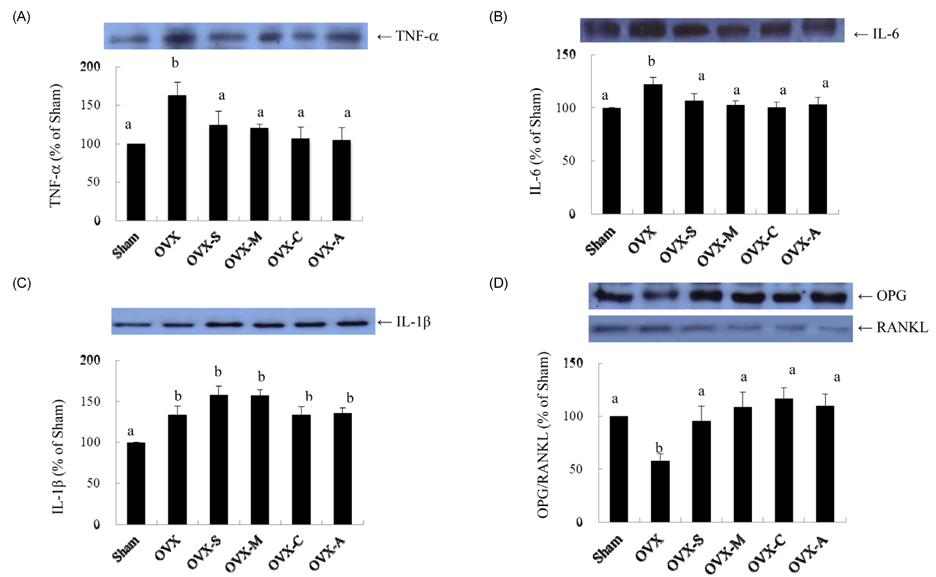
- In previous studies, we found that the consumption of legumes decreased bone turnover in ovariectomized rats. The purpose of the present study is to determine whether the protective effects on bone mineral density (BMD) and the microarchitecture of a diet containing legumes are comparable. In addition, we aim to determine their protective actions in bones by studying bone specific gene expression. Forty-two Sprague-Dawley rats are being divided into six groups during the 12 week study: 1) rats that underwent sham operations (Sham), 2) ovariectomized rats fed an AIN-93M diet (OVX), 3) ovariectomized rats fed an AIN-93M diet with soybeans (OVX-S), 4) ovariectomized rats fed an AIN-93M diet with mung beans (OVX-M), 5) ovariectomized rats fed an AIN-93M diet with cowpeas (OVX-C), and 6) ovariectomized rats fed an AIN-93M diet with azuki beans (OVX-A). Consumption of legumes significantly increased BMD of the spine and femur and bone volume of the femur compared to the OVX. Serum calcium and phosphate ratio, osteocalcin, expression of osteoprotegerin (OPG), and the receptor activator of nuclear factor kappaB ligand (RANKL) ratio increased significantly, while urinary excretion of calcium and deoxypyridinoline and expression of TNF-alpha and IL-6 were significantly reduced in OVX rats fed legumes, compared to OVX rats that were not fed legumes. This study demonstrates that consumption of legumes has a beneficial effect on bone through modulation of OPG and RANKL expression in ovariectomized rats and that legume consumption can help compensate for an estrogen-deficiency by preventing bone loss induced by ovarian hormone deficiency.
MeSH Terms
-
Amino Acids
Animals
Bone Density
Calcium
Cytokines
Diet
Fabaceae
Femur
Gene Expression
Interleukin-6
Osteocalcin
Osteoprotegerin
RANK Ligand
Rats
Rats, Sprague-Dawley
Salicylamides
Soybeans
Spine
Tumor Necrosis Factor-alpha
Amino Acids
Calcium
Cytokines
Interleukin-6
Osteocalcin
Osteoprotegerin
RANK Ligand
Salicylamides
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Ministry of Health and Welfare. Korea Centers for Disease Control and Prevention. The fourth Korea National Health and Nutrition Examination Survey (KNHANES 4). Cheongwon: Korea Centers for Disease Control and Prevention;2009.2. Gallagher JC. The pathogenesis of osteoporosis. Bone Miner. 1990; 9:215–227.
Article3. Stěpán JJ, Pospíchal J, Presl J, Pacovský V. Bone loss and biochemical indices of bone remodeling in surgically induced postmenopausal women. Bone. 1987; 8:279–284.
Article4. Eghbali-Fatourechi G, Khosla S, Sanyal A, Boyle WJ, Lacey DL, Riggs BL. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J Clin Invest. 2003; 111:1221–1230.
Article5. Bord S, Ireland DC, Beavan SR, Compston JE. The effects of estrogen on osteoprotegerin, RANKL, and estrogen receptor expression in human osteoblasts. Bone. 2003; 32:136–141.
Article6. Lacey JV Jr, Mink PJ, Lubin JH, Sherman ME, Troisi R, Hartge P, Schatzkin A, Schairer C. Menopausal hormone replacement therapy and risk of ovarian cancer. JAMA. 2002; 288:334–341.
Article7. Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH. Women's Health Initiative Memory Study. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004; 291:2947–2958.
Article8. Rozman KK, Bhatia J, Calafat AM, Chambers C, Culty M, Etzel RA, Flaws JA, Hansen DK, Hoyer PB, Jeffery EH, Kesner JS, Marty S, Thomas JA, Umbach D. NTP-CERHR expert panel report on the reproductive and developmental toxicity of genistein. Birth Defects Res B Dev Reprod Toxicol. 2006; 77:485–638.
Article9. Delmas PD, Bjarnason NH, Mitlak BH, Ravoux AC, Shah AS, Huster WJ, Draper M, Christiansen C. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N Engl J Med. 1997; 337:1641–1647.
Article10. Cosman F, Lindsay R. Selective estrogen receptor modulators: clinical spectrum. Endocr Rev. 1999; 20:418–434.
Article11. Setchell KD. Soy isoflavones--benefits and risks from nature's selective estrogen receptor modulators (SERMs). J Am Coll Nutr. 2001; 20:354S–362S.
Article12. Picherit C, Bennetau-Pelissero C, Chanteranne B, Lebecque P, Davicco MJ, Barlet JP, Coxam V. Soybean isoflavones dose-dependently reduce bone turnover but do not reverse established osteopenia in adult ovariectomized rats. J Nutr. 2001; 131:723–728.
Article13. Arjmandi BH, Alekel L, Hollis BW, Amin D, Stacewicz-Sapuntzakis M, Guo P, Kukreja SC. Dietary soybean protein prevents bone loss in an ovariectomized rat model of osteoporosis. J Nutr. 1996; 126:161–167.
Article14. Jo HJ, Choi MJ. Effects of isoflavone supplementation on the bone mineral density of growing female rats. Nutr Res Pract. 2008; 2:68–73.
Article15. Zhang Y, Li Q, Wan HY, Helferich WG, Wong MS. Genistein and a soy extract differentially affect three-dimensional bone parameters and bone-specific gene expression in ovariectomized mice. J Nutr. 2009; 139:2230–2236.
Article16. Chen KI, Erh MH, Su NW, Liu WH, Chou CC, Cheng KC. Soyfoods and soybean products: from traditional use to modern applications. Appl Microbiol Biotechnol. 2012; 96:9–22.
Article17. Lee SH, Jin N, Paik DJ, Kim DY, Chung IM, Park Y. Consumption of legumes improves certain bone markers in ovariectomized rats. Nutr Res. 2011; 31:397–403.
Article18. Waarsing JH, Day JS, Weinans H. An improved segmentation method for in vivo microCT imaging. J Bone Miner Res. 2004; 19:1640–1650.
Article19. Arjmandi BH, Birnbaum R, Goyal NV, Getlinger MJ, Juma S, Alekel L, Hasler CM, Drum ML, Hollis BW, Kukreja SC. Bone-sparing effect of soy protein in ovarian hormone-deficient rats is related to its isoflavone content. Am J Clin Nutr. 1998; 68:1364S–1368S.
Article20. Blum SC, Heaton SN, Bowman BM, Hegsted M, Miller SC. Dietary soy protein maintains some indices of bone mineral density and bone formation in aged ovariectomized rats. J Nutr. 2003; 133:1244–1249.
Article21. Cai DJ, Zhao Y, Glasier J, Cullen D, Barnes S, Turner CH, Wastney M, Weaver CM. Comparative effect of soy protein, soy isoflavones, and 17beta-estradiol on bone metabolism in adult ovariectomized rats. J Bone Miner Res. 2005; 20:828–839.
Article22. Fanti P, Monier-Faugere MC, Geng Z, Schmidt J, Morris PE, Cohen D, Malluche HH. The phytoestrogen genistein reduces bone loss in short-term ovariectomized rats. Osteoporos Int. 1998; 8:274–281.
Article23. Byun JS, Lee SS. Effect of soybeans and sword beans on bone metabolism in a rat model of osteoporosis. Ann Nutr Metab. 2010; 56:106–112.
Article24. Wronski TJ, Cintrón M, Doherty AL, Dann LM. Estrogen treatment prevents osteopenia and depresses bone turnover in ovariectomized rats. Endocrinology. 1988; 123:681–686.
Article25. Kalu DN, Liu CC, Salerno E, Hollis B, Echon R, Ray M. Skeletal response of ovariectomized rats to low and high doses of 17 beta-estradiol. Bone Miner. 1991; 14:175–187.
Article26. Choi EM, Suh KS, Kim YS, Choue RW, Koo SJ. Soybean ethanol extract increases the function of osteoblastic MC3T3-E1 cells. Phytochemistry. 2001; 56:733–739.
Article27. Lee YS, Chen X, Anderson JJ. Physiological concentrations of genistein stimulate the proliferation and protect against free radical-induced oxidative damage of MC3T3-E1 osteoblast-like cells. Nutr Res. 2001; 21:1287–1298.
Article28. Chen XW, Garner SC, Anderson JJ. Isoflavones regulate interleukin-6 and osteoprotegerin synthesis during osteoblast cell differentiation via an estrogen-receptor-dependent pathway. Biochem Biophys Res Commun. 2002; 295:417–422.
Article29. Uesugi T, Fukui Y, Yamori Y. Beneficial effects of soybean isoflavone supplementation on bone metabolism and serum lipids in postmenopausal Japanese women: a four-week study. J Am Coll Nutr. 2002; 21:97–102.
Article30. Scheiber MD, Liu JH, Subbiah MT, Rebar RW, Setchell KD. Dietary inclusion of whole soy foods results in significant reductions in clinical risk factors for osteoporosis and cardiovascular disease in normal postmenopausal women. Menopause. 2001; 8:384–392.
Article31. Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997; 89:309–319.
Article32. Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998; 93:165–176.
Article33. Marini H, Minutoli L, Polito F, Bitto A, Altavilla D, Atteritano M, Gaudio A, Mazzaferro S, Frisina A, Frisina N, Lubrano C, Bonaiuto M, D'Anna R, Cannata ML, Corrado F, Cancellieri F, Faraci M, Marini R, Adamo EB, Wilson S, Squadrito F. OPG and sRANKL serum concentrations in osteopenic, postmenopausal women after 2-year genistein administration. J Bone Miner Res. 2008; 23:715–720.
Article34. Marini H, Bitto A, Altavilla D, Burnett BP, Polito F, Di Stefano V, Minutoli L, Atteritano M, Levy RM, D'Anna R, Frisina N, Mazzaferro S, Cancellieri F, Cannata ML, Corrado F, Frisina A, Adamo V, Lubrano C, Sansotta C, Marini R, Adamo EB, Squadrito F. Breast safety and efficacy of genistein aglycone for postmenopausal bone loss: a follow-up study. J Clin Endocrinol Metab. 2008; 93:4787–4796.
Article35. Pfeilschifter J, Köditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002; 23:90–119.
Article36. Ragab AA, Nalepka JL, Bi Y, Greenfield EM. Cytokines synergistically induce osteoclast differentiation: support by immortalized or normal calvarial cells. Am J Physiol Cell Physiol. 2002; 283:C679–C687.
Article37. Gertz ER, Silverman NE, Wise KS, Hanson KB, Alekel DL, Stewart JW, Perry CD, Bhupathiraju SN, Kohut ML, Van Loan MD. Contribution of serum inflammatory markers to changes in bone mineral content and density in postmenopausal women: a 1-year investigation. J Clin Densitom. 2010; 13:277–282.
Article38. Omi N, Aoi S, Murata K, Ezawa I. Evaluation of the effect of soybean milk and soybean milk peptide on bone metabolism in the rat model with ovariectomized osteoporosis. J Nutr Sci Vitaminol (Tokyo). 1994; 40:201–211.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison on New Bone Formation Between Ovariectomized Rats and Normal Rats After Graft of Alloplastic bone material
- Effect of platelet-rich plasma on bone regeneration in ovariectomized osteoporotic rats
- Effects of Bone Density as Injections of Salmon Calcitonin Micropheres in Ovariectomized Rats
- The Effects Of 17 beta-Estradiol On Osseous Tissue In Ovariectomized Rats
- Changes in the Expression Pattern of Cyclooxygenase-2, Mapkinases and Related Apoptotic Markers by Different Levels of Estrogen Supplementation in Mature or Ovariectomized Female Rat Heart