Nutr Res Pract.
2013 Feb;7(1):26-33.
Lycopene supplementation suppresses oxidative stress induced by a high fat diet in gerbils
- Affiliations
-
- 1Department of Food and Nutrition, Yeungnam University, 214-1, Dae-dong, Gyeongsan, Gyeongbuk 712-749, Korea. Jsseo@ynu.ac.kr
Abstract
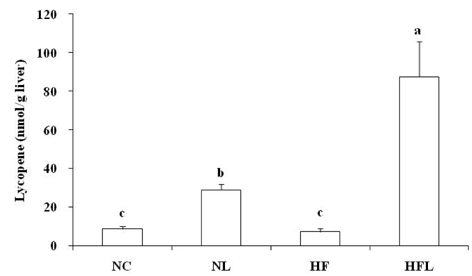
- The effect of lycopene supplementation on the antioxidant system was investigated by analyzing lipid peroxide levels, glutathione contents, and antioxidant enzyme activities in Mongolian gerbils fed a high fat diet. Gerbils were fed on each experimental diet for 6 weeks; normal diet (NC), normal diet with 0.05% lycopene (NL), high fat diet (HF), and a high fat diet with 0.05% lycopene (HFL). Dietary supplementation of lycopene increased hepatic lycopene level in gerbils fed a normal or high fat diet (P < 0.05). Liver and erythrocyte concentrations of lipid peroxide increased in gerbils fed a high fat diet, whereas lycopene supplementation decreased liver and erythrocyte concentrations of lipid peroxide (P < 0.05). Hepatic total glutathione content was higher in the NL group than that in the NC group (P < 0.05). Total antioxidant status in plasma increased following lycopene supplementation compared with that of the non-lycopene supplemented groups (P < 0.05). Hepatic catalase activity increased following dietary lycopene supplementation (P < 0.05). Superoxide dismutase activity in liver remained unchanged with lycopene supplementation, but erythrocyte superoxide dismutase activity increased in NL group compared with NC group (P < 0.05). Glutathione-S-transferase activity increased in the NL group compared to NC group (P < 0.05). Liver and erythrocyte glutathione peroxidase activity increased significantly in the NL group compared to that in the HF group (P < 0.05). Liver glutathione reductase activity was higher in the NL group than that in the NC group (P < 0.05). These results suggest that lycopene supplementation may be efficient for preventing chronic diseases induced by oxidative stress related to high fat diet.
Keyword
MeSH Terms
Figure
Reference
-
1. Halliwell B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006. 141:312–322.
Article2. Bray GA, Paeratakul S, Popkin BM. Dietary fat and obesity: a review of animal, clinical and epidemiological studies. Physiol Behav. 2004. 83:549–555.
Article3. Spector A. Review: oxidative stress and disease. J Ocul Pharmacol Ther. 2000. 16:193–201.
Article4. Rudich A, Kanety H, Bashan N. Adipose stress-sensing kinases: linking obesity to malfunction. Trends Endocrinol Metab. 2007. 18:291–299.
Article5. Blokhina O, Virolainen E, Fagerstedt KV. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot. 2003. 91 Spec No:179–194.
Article6. Martínez A, Rodríguez-Gironés MA, Barbosa A, Costas M. Donator acceptor map for carotenoids, melatonin and vitamins. J Phys Chem A. 2008. 112:9037–9042.
Article7. Lauretani F, Semba RD, Dayhoff-Brannigan M, Corsi AM, Di Iorio A, Buiatti E, Bandinelli S, Guralnik JM, Ferrucci L. Low total plasma carotenoids are independent predictors of mortality among older persons: the InCHIANTI study. Eur J Nutr. 2008. 47:335–340.
Article8. Omoni AO, Aluko RE. The anti-carcinogenic and anti-atherogenic effects of lycopene: a review. Trends Food Sci Technol. 2005. 16:344–350.
Article9. Ellinger S, Ellinger J, Müller SC, Stehle P. Tomatoes and lycopene in prevention and therapy--is there an evidence for prostate diseases? Aktuelle Urol. 2009. 40:37–43.10. Amin AR, Kucuk O, Khuri FR, Shin DM. Perspectives for cancer prevention with natural compounds. J Clin Oncol. 2009. 27:2712–2725.
Article11. Gupta SK, Trivedi D, Srivastava S, Joshi S, Halder N, Verma SD. Lycopene attenuates oxidative stress induced experimental cataract development: an in vitro and in vivo study. Nutrition. 2003. 19:794–799.
Article12. Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004. 114:1752–1761.
Article13. Castenmiller JJ, West CE. Bioavailability and bioconversion of carotenoids. Annu Rev Nutr. 1998. 18:19–38.
Article14. Lee CM, Lederman JD, Hofmann NE, Erdman JW. The Mongolian gerbil (Meriones unguiculatus) is an appropriate animal model for evaluation of the conversion of β-carotene to vitamin A. J Nutr. 1998. 128:280–286.
Article15. Huang CS, Chuang CH, Hu ML. Effects of lycopene supplementation on plasma and tissue lycopene levels in various rodent strains. Int J Vitam Nutr Res. 2006. 76:377–384.
Article16. Boileau TW, Clinton SK, Erdman JW Jr. Tissue lycopene concentrations and isomer patterns are affected by androgen status and dietary lycopene concentration in male F344 rats. J Nutr. 2000. 130:1613–1618.
Article17. Jain CK, Agarwal S, Rao AV. The effect of dietary lycopene on bioavailability, tissue distribution, in vivo antioxidant properties and colonic preneoplasia in rats. Nutr Res. 1999. 19:1383–1391.
Article18. Hogeboom GH. [3] Fractionation of cell components of animal tissues. Methods Enzymol. 1955. 1:16–19.
Article19. Miller KW, Lorr NA, Yang CS. Simultaneous determination of plasma retinol, α-tocopherol, lycopene, α-carotene, and β-carotene by high-performance liquid chromatography. Anal Biochem. 1984. 138:340–345.
Article20. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979. 95:351–358.
Article21. Aebi H. Bergmeyer HU, Gawehn K, editors. Catalase. Methods of Enzymatic Analysis. 1974. 2nd ed. New York: Academic Press;673–689.
Article22. Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974. 47:469–474.
Article23. Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967. 70:158–169.24. Pinto RE, Bartley W. The effect of age and sex on glutathione reductase and glutathione peroxidase activities and on aerobic glutathione oxidation in rat liver homogenates. Biochem J. 1969. 112:109–115.
Article25. Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974. 249:7130–7139.26. Rao AV. Lycopene, tomatoes, and the prevention of coronary heart disease. Exp Biol Med (Maywood). 2002. 227:908–913.
Article27. Agarwal S, Rao AV. Tomato lycopene and its role in human health and chronic diseases. CMAJ. 2000. 163:739–744.28. Mackinnon ES, Rao AV, Josse RG, Rao LG. Supplementation with the antioxidant lycopene significantly decreases oxidative stress parameters and the bone resorption marker N-telopeptide of type I collagen in postmenopausal women. Osteoporos Int. 2011. 22:1091–1101.
Article29. Agarwal S, Rao AV. Tomato lycopene and low density lipoprotein oxidation: a human dietary intervention study. Lipids. 1998. 33:981–984.
Article30. Kravchenko LV, Morozov SV, Beketova NA, Deryagina VP, Avren'eva LI, Tutel'yan VA. Antioxidant status of rats receiving lycopene in different doses. Bull Exp Biol Med. 2003. 135:353–357.31. Gitenay D, Lyan B, Rambeau M, Mazur A, Rock E. Comparison of lycopene and tomato effects on biomarkers of oxidative stress in vitamin E deficient rats. Eur J Nutr. 2007. 46:468–475.
Article32. Tso P, Lee T, DeMichele SJ. Randomized structured triglycerides increase lymphatic absorption of tocopherol and retinol compared with the equivalent physical mixture in a rat model of fat malabsorption. J Nutr. 2001. 131:2157–2163.
Article33. Lee A, Thurnham DI, Chopra M. Consumption of tomato products with olive oil but not sunflower oil increases the antioxidant activity of plasma. Free Radic Biol Med. 2000. 29:1051–1055.
Article34. Unlu NZ, Bohn T, Clinton SK, Schwartz SJ. Carotenoid absorption from salad and salsa by humans is enhanced by the addition of avocado or avocado oil. J Nutr. 2005. 135:431–436.
Article35. Requena JR, Fu MX, Ahmed MU, Jenkins AJ, Lyons TJ, Thorpe SR. Lipoxidation products as biomarkers of oxidative damage to proteins during lipid peroxidation reactions. Nephrol Dial Transplant. 1996. 11:Suppl 5. 48–53.
Article36. Hegsted DM, Gallagher A. Dietary fat and cholesterol and serum cholesterol in the gerbil. J Lipid Res. 1967. 8:210–214.
Article37. Bahcecioglu IH, Kuzu N, Metin K, Ozercan IH, Ustündag B, Sahin K, Kucuk O. Lycopene prevents development of steatohepatitis in experimental nonalcoholic steatohepatitis model induced by high-fat diet. Vet Med Int. 2010. 2010:pii: 262179.
Article38. Ono H, Sakamoto A, Sakura N. Plasma total glutathione concentrations in healthy pediatric and adult subjects. Clin Chim Acta. 2001. 312:227–229.
Article39. Paolicchi A, Dominici S, Pieri L, Maellaro E, Pompella A. Glutathione catabolism as a signaling mechanism. Biochem Pharmacol. 2002. 64:1027–1035.
Article40. Hsu CL, Yen GC. Effect of gallic acid on high fat diet-induced dyslipidaemia, hepatosteatosis and oxidative stress in rats. Br J Nutr. 2007. 98:727–735.
Article41. Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW 3rd, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009. 119:573–581.
Article42. Leal M, Shimada A, Ruíz F, González de Mejía E. Effect of lycopene on lipid peroxidation and glutathione-dependent enzymes induced by T-2 toxin in vivo. Toxicol Lett. 1999. 109:1–10.
Article43. Moreira EA, Fagundes RL, Filho DW, Neves D, Sell F, Bellisle F, Kupek E. Effects of diet energy level and tomato powder consumption on antioxidant status in rats. Clin Nutr. 2005. 24:1038–1046.
Article44. Kumar P, Kumar A. Effect of lycopene and epigallocatechin-3-gallate against 3-nitropropionic acid induced cognitive dysfunction and glutathione depletion in rat: a novel nitric oxide mechanism. Food Chem Toxicol. 2009. 47:2522–2530.
Article45. Hsu CL, Wu CH, Huang SL, Yen GC. Phenolic compounds rutin and o-coumaric acid ameliorate obesity induced by high-fat diet in rats. J Agric Food Chem. 2009. 57:425–431.
Article46. Lee SJ, Choi SK, Seo JS. Grape skin improves antioxidant capacity in rats fed a high fat diet. Nutr Res Pract. 2009. 3:279–285.
Article47. Atessahin A, Yilmaz S, Karahan I, Ceribasi AO, Karaoglu A. Effects of lycopene against cisplatin-induced nephrotoxicity and oxidative stress in rats. Toxicology. 2005. 212:116–123.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of vitamin C and E supplementation on oxidative stress and liver toxicity in rats fed a low-fat ethanol diet
- Effect of Dietary Fat and Genistein on Lipid Metabolism and Antioxidant Activity in Hyperlipidemic Male Rats induced High Fat Diet
- Effects of d-alpha-tocopherol supplements on lipid metabolism in a high-fat diet-fed animal model
- Antioxidant effects of fucoxanthin rich powder in rats fed with high fat diet
- Suppression of oxidative stress by grape seed supplementation in rats