Nutr Res Pract.
2013 Dec;7(6):475-480.
Antioxidant effects of fucoxanthin rich powder in rats fed with high fat diet
- Affiliations
-
- 1Department of Food Science and Nutrition, Dankook University, 126 Jukjeon-dong, Suji-gu, Yongin, Gyunggi 448-701, Korea. wkkim@dankook.ac.kr
Abstract
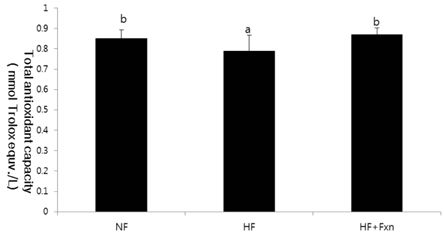
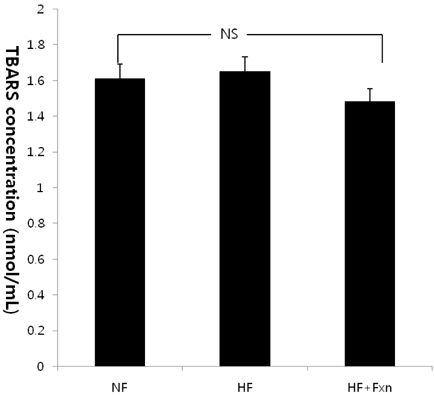
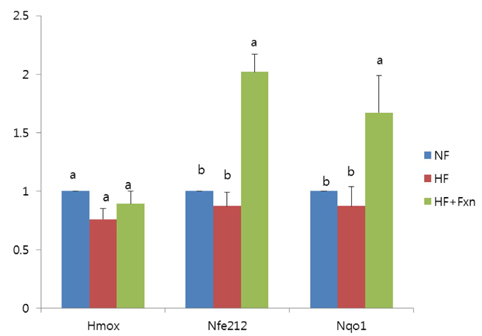
- The purpose of this study was to determine the antioxidant effect of fucoxanthin. After rats were fed a normal fat diet (NF), high fat diet (HF), and high fat with 0.2% fucoxanthin diet (HF + Fxn) for 4 weeks, the markers of oxidative stress and antioxidant capacity like lipid peroxidation, plasma total antioxidant capacity (TAC), and activities of antioxidant enzymes (catalase, superoxide dismutase (SOD), and gluthathione peroxidase (GSH-Px)) were determined. mRNA expression of transcription factor, nuclear erythroid factor like 2 (Nrf2), and its target genes such as NAD(P)H quinone oxidoreductase1 (NQO1) and heme oxygenase-1 (HO-1) were also determined. Mean weight gain in the HF + Fxn group was lower, without statistical significance, and the total food intake in the HF + Fxn group was lower than that in the HF group (P < 0.05). The activity of GSH-Px (P < 0.05) in plasma was significantly higher in the HF + Fxn group than those in the HF group (P < 0.05). In the liver, the activities of catalase (P < 0.05) and GSH-Px (P < 0.05) in the HF + Fxn group were significantly higher than those in the HF group. Plasma TAC level was significantly higher in the HF + Fxn group than that in the HF group (P < 0.05). Lipid peroxidation in plasma tended to be lower without statistical significance. Fucoxanthin supplements were shown to have higher mRNA expression of Nrf2 and NQO1 than those in the high fat diet only group (P < 0.05). In conclusion, supplementation of fucoxanthin improved the antioxidant capacity, depleted by high fat diet, by activating the Nrf2 pathway and its downstream target gene NQO1. Therefore, supplementation of fucoxanthin, especially for those who consume high fat in their diet, may benefit from reduced risk of oxidative stress.
Keyword
MeSH Terms
-
Animals
Antioxidants*
Benzoquinones
Catalase
Diet
Diet, High-Fat*
Eating
Heme Oxygenase-1
Lipid Peroxidation
Liver
Oxidative Stress
Peroxidase
Plasma
Rats*
RNA, Messenger
Superoxide Dismutase
Transcription Factors
Weight Gain
Xanthophylls
Antioxidants
Benzoquinones
Catalase
Heme Oxygenase-1
Peroxidase
RNA, Messenger
Superoxide Dismutase
Transcription Factors
Xanthophylls
Figure
Reference
-
1. Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation. 2005; 111:1448–1454.2. Milagro FI, Campión J, Martínez JA. Weight gain induced by high-fat feeding involves increased liver oxidative stress. Obesity (Silver Spring). 2006; 14:1118–1123.
Article3. Uzun H, Zengin K, Taskin M, Aydin S, Simsek G, Dariyerli N. Changes in leptin, plasminogen activator factor and oxidative stress in morbidly obese patients following open and laparoscopic Swedish adjustable gastric banding. Obes Surg. 2004; 14:659–665.
Article4. Kwak MK, Itoh K, Yamamoto M, Sutter TR, Kensler TW. Role of transcription factor Nrf2 in the induction of hepatic phase 2 and antioxidative enzymes in vivo by the cancer chemoprotective agent, 3H-1, 2-dimethiole-3-thione. Mol Med. 2001; 7:135–145.
Article5. Cho HY, Jedlicka AE, Reddy SP, Kensler TW, Yamamoto M, Zhang LY, Kleeberger SR. Role of NRF2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol. 2002; 26:175–182.
Article6. Hsu CL, Yen GC. Phenolic compounds: evidence for inhibitory effects against obesity and their underlying molecular signaling mechanisms. Mol Nutr Food Res. 2008; 52:53–61.
Article7. Maeda H, Tsukui T, Sashima T, Hosokawa M, Miyashita K. Seaweed carotenoid, fucoxanthin, as a multi-functional nutrient. Asia Pac J Clin Nutr. 2008; 17:Suppl 1. 196–199.8. Sangeetha RK, Bhaskar N, Baskaran V. Comparative effects of beta-carotene and fucoxanthin on retinol deficiency induced oxidative stress in rats. Mol Cell Biochem. 2009; 331:59–67.
Article9. Airanthi MK, Hosokawa M, Miyashita K. Comparative antioxidant activity of edible Japanese brown seaweeds. J Food Sci. 2011; 76:C104–C111.
Article10. Sachindra NM, Sato E, Maeda H, Hosokawa M, Niwano Y, Kohno M, Miyashita K. Radical scavenging and singlet oxygen quenching activity of marine carotenoid fucoxanthin and its metabolites. J Agric Food Chem. 2007; 55:8516–8522.
Article11. Kumar CS, Ganesan P, Suresh PV, Bhaskar N. Seaweeds as a source of nutritionally beneficial compounds-a review. J Food Sci Technol. 2008; 45:1–13.12. Khan MN, Choi JS, Lee MC, Kim E, Nam TJ, Fujii H, Hong YK. Anti-inflammatory activities of methanol extracts from various seaweed species. J Environ Biol. 2008; 29:465–469.13. Kotake-Nara E, Kushiro M, Zhang H, Sugawara T, Miyashita K, Nagao A. Carotenoids affect proliferation of human prostate cancer cells. J Nutr. 2001; 131:3303–3306.
Article14. Liu CL, Liang AL, Hu ML. Protective effects of fucoxanthin against ferric nitrilotriacetate-induced oxidative stress in murine hepatic BNL CL.2 cells. Toxicol In Vitro. 2011; 25:1314–1319.
Article15. Liu CL, Chiu YT, Hu ML. Fucoxanthin enhances HO-1 and NQO1 expression in murine hepatic BNL CL.2 cells through activation of the Nrf2/ARE system partially by its pro-oxidant activity. J Agric Food Chem. 2011; 59:11344–11351.
Article16. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957; 226:497–509.
Article17. Santos MT, Valles J, Aznar J, Vilches J. Determination of plasma malondialdehyde-like material and its clinical application in stroke patients. J Clin Pathol. 1980; 33:973–976.
Article18. Miller NJ, Rice-Evans CA. Factors influencing the antioxidant activity determined by the ABTS.+ radical cation assay. Free Radic Res. 1997; 26:195–199.
Article19. Rice-Evans CA. Measurement of total antioxidant activity as a marker of antioxidant status in vivo: procedures and limitations. Free Radic Res. 2000; 33:Suppl. S59–S66.20. Aebi H. Catalase in vitro. Methods Enzymol. 1984; 105:121–126.21. Fridovich I. Superoxide radical: an endogenous toxicant. Annu Rev Pharmacol Toxicol. 1983; 23:239–257.
Article22. Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967; 70:158–169.23. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193:265–275.
Article24. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25:402–408.
Article25. Wang YP, Cheng ML, Zhang BF, Mu M, Wu J. Effects of blueberry on hepatic fibrosis and transcription factor Nrf2 in rats. World J Gastroenterol. 2010; 16:2657–2663.
Article26. Ueda K, Ueyama T, Yoshida K, Kimura H, Ito T, Shimizu Y, Oka M, Tsuruo Y, Ichinose M. Adaptive HNE-Nrf2-HO-1 pathway against oxidative stress is associated with acute gastric mucosal lesions. Am J Physiol Gastrointest Liver Physiol. 2008; 295:G460–G469.
Article27. Park HJ, Lee MK, Park YB, Shin YC, Choi MS. Beneficial effects of undaria pinnatifida ethanol extract on diet-induced-insulin resistance in C57BL/6J mice. Food Chem Toxicol. 2011; 49:727–733.
Article28. Maeda H, Hosokawa M, Sashima T, Miyashita K. Dietary combination of fucoxanthin and fish oil attenuates the weight gain of white adipose tissue and decreases blood glucose in obese/diabetic KK-Ay mice. J Agric Food Chem. 2007; 55:7701–7706.
Article29. Rizvi SI, Maurya PK. Markers of oxidative stress in erythrocytes during aging in humans. Ann N Y Acad Sci. 2007; 1100:373–382.
Article30. Muthulakshmi S, Saravanan R. Protective effects of azelaic acid against high-fat diet-induced oxidative stress in liver, kidney and heart of C57BL/6J mice. Mol Cell Biochem. 2013; 377:23–33.
Article31. Dai FJ, Hsu WH, Huang JJ, Wu SC. Effect of pigeon pea (Cajanus cajan L.) on high-fat diet-induced hypercholesterolemia in hamsters. Food Chem Toxicol. 2013; 53:384–391.
Article32. Heo SJ, Jeon YJ. Protective effect of fucoxanthin isolated from Sargassum siliquastrum on UV-B induced cell damage. J Photochem Photobiol B. 2009; 95:101–107.
Article33. Ravi Kumar S, Narayan B, Vallikannan B. Fucoxanthin restrains oxidative stress induced by retinol deficiency through modulation of Na(+)K(+)-ATPase and antioxidant enzyme activities in rats. Eur J Nutr. 2008; 47:432–441.
Article34. Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997; 236:313–322.
Article35. Valerio LG Jr, Kepa JK, Pickwell GV, Quattrochi LC. Induction of human NAD(P)H:quinone oxidoreductase (NQO1) gene expression by the flavonol quercetin. Toxicol Lett. 2001; 119:49–57.
Article36. Yang C, Zhang X, Fan H, Liu Y. Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res. 2009; 1282:133–141.
Article37. Tanaka Y, Aleksunes LM, Yeager RL, Gyamfi MA, Esterly N, Guo GL, Klaassen CD. NF-E2-related factor 2 inhibits lipid accumulation and oxidative stress in mice fed a high-fat diet. J Pharmacol Exp Ther. 2008; 325:655–664.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Dietary Grape Pomace on Lipid Oxidation and Related Enzyme Activities in Rats Fed High Fat Diet
- Grape skin improves antioxidant capacity in rats fed a high fat diet
- The effect of fucoxanthin rich power on the lipid metabolism in rats with a high fat diet
- The effects of Angelica keiskei Koidz on the expression of antioxidant enzymes related to lipid profiles in rats fed a high fat diet
- Effects of Acorn Supplementation on Lipid Profiles and Antioxidant Enzyme Activities in High Fat Diet-Induced Obese Rats