Nutr Res Pract.
2012 Aug;6(4):286-293.
Anti-obesity effect of resveratrol-amplified grape skin extracts on 3T3-L1 adipocytes differentiation
- Affiliations
-
- 1Department of Food and Nutrition, Yeungnam University, 214-1, Dae-dong, Gyeongsan-si, Gyeongbuk 712-749, Korea. jsseo@ynu.ac.kr
- 2Samsung Biomedical Research Institute, Seoul 135-710, Korea.
Abstract
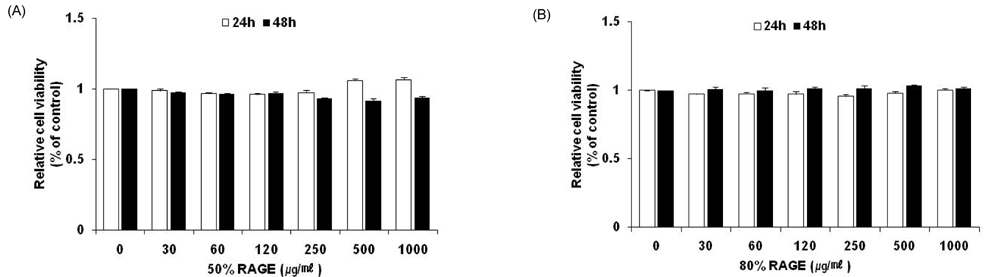
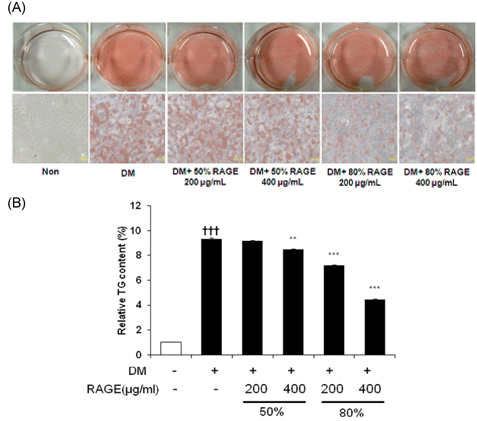
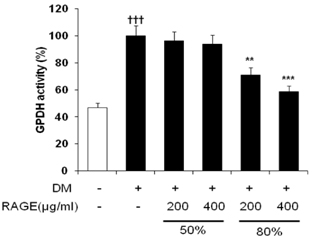
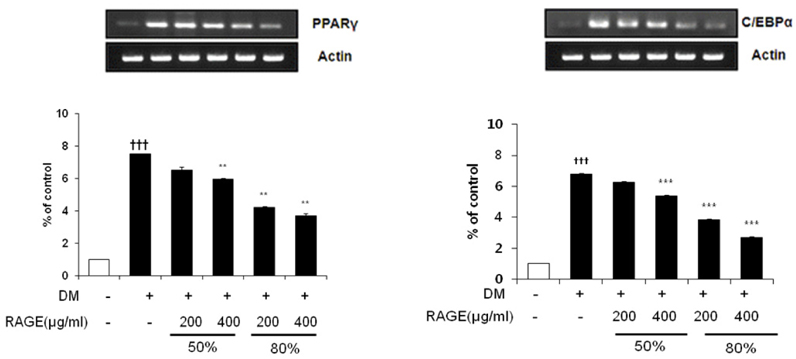
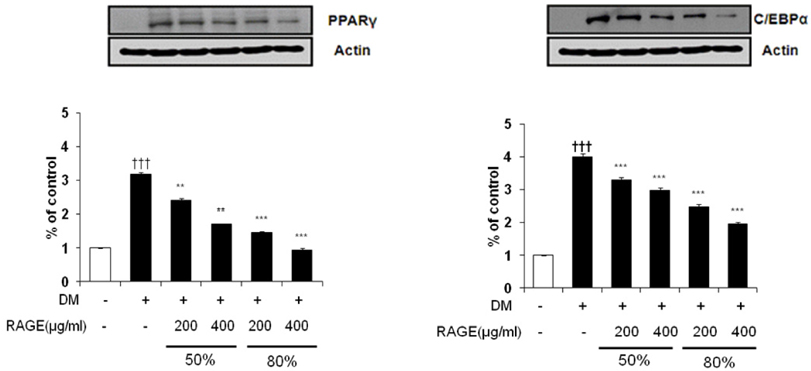
- Resveratrol (3,4,5-trihydroxy-trans-stilbene), a phytoalexin found in grape skin, grape products, and peanuts as well as red wine, has been reported to have various biological and pharmacological properties. The purpose of this study was to investigate the anti-obesity effect of resveratrol-amplified grape skin extracts on adipocytes. The anti-obesity effects of grape skin extracts were investigated by measuring proliferation and differentiation in 3T3-L1 cells. The effect of grape skin ethanol extracts on cell proliferation was detected by the MTS assay. The morphological changes and degree of adipogenesis of preadipocyte 3T3-L1 cells were measured by Oil Red-O staining assay. Treatment with extracts of resveratrol-amplified grape skin decreased lipid accumulation and glycerol-3-phosphate dehydrogenase activity without affecting 3T3-L1 cell viability. Grape skin extract treatment resulted in significantly attenuated expression of key adipogenic transcription factors, including peroxisome proliferator-activated receptor, CCAAT/enhancer-binding proteins, and their target genes (FAS, aP2, SCD-1, and LPL). These results indicate that resveratrol-amplified grape skin extracts may be useful for preventing obesity by regulating lipid metabolism.
MeSH Terms
Figure
Reference
-
1. Szkudelska K, Szkudelski T. Resveratrol, obesity and diabetes. Eur J Pharmacol. 2010. 635:1–8.
Article2. Das DK, Maulik N. Resveratrol in cardioprotection: a therapeutic promise of alternative medicine. Mol Interv. 2006. 6:36–47.
Article3. Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell. 2006. 127:1109–1122.
Article4. Filip V, Plockova M, Smidrkal J, Spickova Z, Melzoch K, Schmidt S. Resveratrol and its antioxidant and antimicrobial effectiveness. Food Chem. 2003. 83:585–593.
Article5. Bradamante S, Barenghi L, Villa A. Cardiovascular protective effects of resveratrol. Cardiovasc Drug Rev. 2004. 22:169–188.
Article6. Athar M, Back JH, Tang X, Kim KH, Kopelovich L, Bickers DR, Kim AL. Resveratrol: a review of preclinical studies for human cancer prevention. Toxicol Appl Pharmacol. 2007. 224:274–283.
Article7. de la Lastra CA, Villegas I. Resveratrol as an antioxidant and pro-oxidant agent: mechanisms and clinical implications. Biochem Soc Trans. 2007. 35:1156–1160.
Article8. Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006. 444:337–342.
Article9. Malterud K, Tonstad S. Preventing obesity: challenges and pitfalls for health promotion. Patient Educ Couns. 2009. 76:254–259.
Article10. Dewulf EM, Cani PD, Neyrinck AM, Possemiers S, Van Holle A, Muccioli GG, Deldicque L, Bindels LB, Pachikian BD, Sohet FM, Mignolet E, Francaux M, Larondelle Y, Delzenne NM. Inulin-type fructans with prebiotic properties counteract GPR43 overexpression and PPARγ-related adipogenesis in the white adipose tissue of high-fat diet-fed mice. J Nutr Biochem. 2011. 22:712–722.
Article11. Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, Shimomura I. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007. 56:901–911.
Article12. Hao HD, He LR. Mechanisms of cardiovascular protection by resveratrol. J Med Food. 2004. 7:290–298.
Article13. Leifert WR, Abeywardena MY. Cardioprotective actions of grape polyphenols. Nutr Res. 2008. 28:729–737.
Article14. Bertelli AA, Das DK. Grapes, wines, resveratrol, and heart health. J Cardiovasc Pharmacol. 2009. 54:468–476.
Article15. Langcake P, Pryce RJ. The production of resveratrol and the viniferins by grapevines in response to ultraviolet irradiation. Phytochemistry. 1977. 16:1193–1196.
Article16. Cho YJ, Maeng JS, Kim CT, Pyee J. Enrichment of resveratrol content in harvested grape using modulation of cell metabolism with UV treatment. J East Asian Soc Diet Life. 2011. 21:739–745.17. Singleton VL, Rossi JA Jr. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965. 16:144–158.18. Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999. 26:1231–1237.
Article19. Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010. 303:235–241.
Article20. Lee CH, Olson P, Evans RM. Minireview: lipid metabolism, metabolic diseases, and peroxisome proliferator-activated receptors. Endocrinology. 2003. 144:2201–2207.
Article21. Harper CE, Patel BB, Wang J, Arabshahi A, Eltoum IA, Lamartiniere CA. Resveratrol suppresses prostate cancer progression in transgenic mice. Carcinogenesis. 2007. 28:1946–1953.
Article22. Bae HS. Effect of grape skin with resveratrol amplification on lipid metabolism and antioxidative system in rats fed high cholesterol diet [master's thesis]. 2008. Gyeongsan: Yeungnam University.23. Wise LS, Green H. Participation of one isozyme of cytosolic glycerophosphate dehydrogenase in the adipose conversion of 3T3 cells. J Biol Chem. 1979. 254:273–275.
Article24. Park HJ, Yang JY, Ambati S, Della-Fera MA, Hausman DB, Rayalam S, Baile CA. Combined effects of genistein, quercetin, and resveratrol in human and 3T3-L1 adipocytes. J Med Food. 2008. 11:773–783.
Article25. Rayalam S, Yang JY, Ambati S, Della-Fera MA, Baile CA. Resveratrol induces apoptosis and inhibits adipogenesis in 3T3-L1 adipocytes. Phytother Res. 2008. 22:1367–1371.
Article26. Szatmari I, Rajnavolgyi E, Nagy L. PPARgamma, a lipid-activated transcription factor as a regulator of dendritic cell function. Ann N Y Acad Sci. 2006. 1088:207–218.
Article27. Zhang L, Chawla A. Role of PPARγ in macrophage biology and atherosclerosis. Trends Endocrinol Metab. 2004. 15:500–505.
Article28. Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell. 1994. 79:1147–1156.
Article29. Tang QQ, Zhang JW, Lane MD. Sequential gene promoter interactions of C/EBPβ, C/EBPα, and PPARγ during adipogenesis. Biochem Biophys Res Commun. 2004. 319:235–239.
Article30. Wu Z, Rosen ED, Brun R, Hauser S, Adelmant G, Troy AE, McKeon C, Darlington GJ, Spiegelman BM. Cross-regulation of C/EBPα and PPARγ controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol Cell. 1999. 3:151–158.
Article31. Yang JY, Della-Fera MA, Rayalam S, Ambati S, Hartzell DL, Park HJ, Baile CA. Enhanced inhibition of adipogenesis and induction of apoptosis in 3T3-L1 adipocytes with combinations of resveratrol and quercetin. Life Sci. 2008. 82:1032–1039.
Article32. Floyd ZE, Wang ZQ, Kilroy G, Cefalu WT. Modulation of peroxisome proliferator-activated receptor γ stability and transcriptional activity in adipocytes by resveratrol. Metabolism. 2008. 57:S32–S38.
Article33. Miles PD, Barak Y, He W, Evans RM, Olefsky JM. Improved insulin-sensitivity in mice heterozygous for PPAR-gamma deficiency. J Clin Invest. 2000. 105:287–292.34. Chen LL, Zhang HH, Zheng J, Hu X, Kong W, Hu D, Wang SX, Zhang P. Resveratrol attenuates high-fat diet-induced insulin resistance by influencing skeletal muscle lipid transport and subsarcolemmal mitochondrial β-oxidation. Metabolism. 2011. 60:1598–1609.
Article35. Fajas L, Schoonjans K, Gelman L, Kim JB, Najib J, Martin G, Fruchart JC, Briggs M, Spiegelman BM, Auwerx J. Regulation of peroxisome proliferator-activated receptor γ expression by adipocyte differentiation and determination factor 1/sterol regulatory element binding protein 1: implications for adipocyte differentiation and metabolism. Mol Cell Biol. 1999. 19:5495–5503.
Article36. Payne VA, Au WS, Lowe CE, Rahman SM, Friedman JE, O'Rahilly S, Rochford JJ. C/EBP transcription factors regulate SREBP1c gene expression during adipogenesis. Biochem J. 2010. 425:215–223.
Article37. Le Lay S, Lefrère I, Trautwein C, Dugail I, Krief S. Insulin and sterol-regulatory element-binding protein-1c (SREBP-1C) regulation of gene expression in 3T3-L1 adipocytes. Identification of CCAAT/enhancer-binding protein beta as an SREBP-1C target. J Biol Chem. 2002. 277:35625–35634.38. Gosmain Y, Dif N, Berbe V, Loizon E, Rieusset J, Vidal H, Lefai E. Regulation of SREBP-1 expression and transcriptional action on HKII and FAS genes during fasting and refeeding in rat tissues. J Lipid Res. 2005. 46:697–705.
Article39. Bennett MK, Lopez JM, Sanchez HB, Osborne TF. Sterol regulation of fatty acid synthase promoter. Coordinate feedback regulation of two major lipid pathways. J Biol Chem. 1995. 270:25578–25583.40. Kim JB, Spiegelman BM. ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Genes Dev. 1996. 10:1096–1107.
Article41. Horton JD, Shimomura I, Brown MS, Hammer RE, Goldstein JL, Shimano H. Activation of cholesterol synthesis in preference to fatty acid synthesis in liver and adipose tissue of transgenic mice overproducing sterol regulatory element-binding protein-2. J Clin Invest. 1998. 101:2331–2339.
Article42. Makowski L, Brittingham KC, Reynolds JM, Suttles J, Hotamisligil GS. The fatty acid-binding protein, aP2, coordinates macrophage cholesterol trafficking and inflammatory activity. Macrophage expression of aP2 impacts peroxisome proliferator-activated receptor gamma and IkappaB kinase activities. J Biol Chem. 2005. 280:12888–12895.
Article43. Makowski L, Hotamisligil GS. Fatty acid binding proteins--the evolutionary crossroads of inflammatory and metabolic responses. J Nutr. 2004. 134:2464S–2468S.
Article44. Kong CS, Kim JA, Ahn BN, Vo TS, Yoon NY, Kim SK. 1-(3',5'-dihydroxyphenoxy)-7-(2",4",6-trihydroxyphenoxy)-2,4,9-t rihydroxydibenzo-1,4-dioxin inhibits adipocyte differentiation of 3T3-L1 fibroblasts. Mar Biotechnol (NY). 2010. 12:299–307.
Article45. Bulló M, García-Lorda P, Peinado-Onsurbe J, Hernández M, Del Castillo D, Argilés JM, Salas-Salvadó J. TNFα expression of subcutaneous adipose tissue in obese and morbid obese females: relationship to adipocyte LPL activity and leptin synthesis. Int J Obes Relat Metab Disord. 2002. 26:652–658.
Article46. Cohen P, Friedman JM. Leptin and the control of metabolism: role for stearoyl-CoA desaturase-1 (SCD-1). J Nutr. 2004. 134:2455S–2463S.
Article47. Cohen P, Miyazaki M, Socci ND, Hagge-Greenberg A, Liedtke W, Soukas AA, Sharma R, Hudgins LC, Ntambi JM, Friedman JM. Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science. 2002. 297:240–243.
Article48. Jeong YS, Jung HK, Cho KH, Youn KS, Hong JH. Anti-obesity effect of grape skin extract in 3T3-L1 adipocytes. Food Sci Biotechnol. 2011. 20:635–642.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pear pomace water extract inhibits adipogenesis and induces apoptosis in 3T3-L1 adipocytes
- Cryptotanshinone Inhibits Lipid Accumulation in Differentiating 3T3-L1 Preadipocytes by Down-regulating C/EBP-α, PPAR-γ, FAS, Perilipin A, and STAT-3
- Cryptotanshinone Inhibits Lipid Accumulation in Differentiating 3T3-L1 Preadipocytes by Down-regulating C/EBP-α, PPAR-γ, FAS, Perilipin A, and STAT-3
- Cryptotanshinone Inhibits Lipid Accumulation in Differentiating 3T3-L1 Preadipocytes by Down-regulating C/EBP-α, PPAR-γ, FAS, Perilipin A, and STAT-3
- Carnosic acid inhibits TLR4-MyD88 signaling pathway in LPS-stimulated 3T3-L1 adipocytes