Nutr Res Pract.
2011 Jun;5(3):198-204.
Herbal extract THI improves metabolic abnormality in mice fed a high-fat diet
- Affiliations
-
- 1Department of Biological Science, Sookmyung Women's University, Cheongpa-ro 47-gil 100, Yongsan-gu, Seoul 140-742, Korea. yyang@sookmyung.ac.kr
- 2Department of Microbiology, College of Medicine, Chung-Ang University, Seoul 156-756, Korea.
Abstract
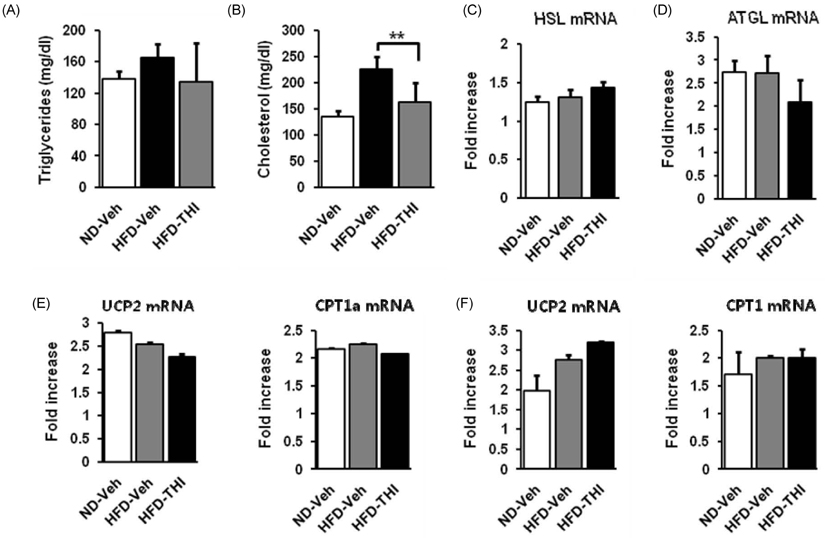
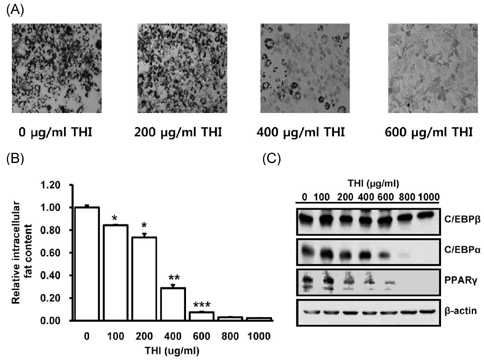
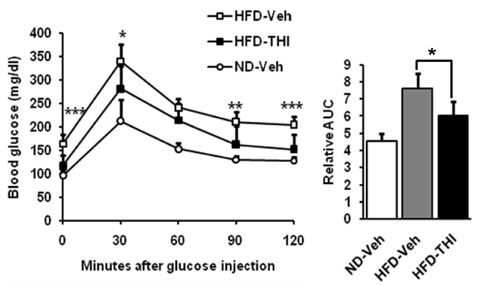
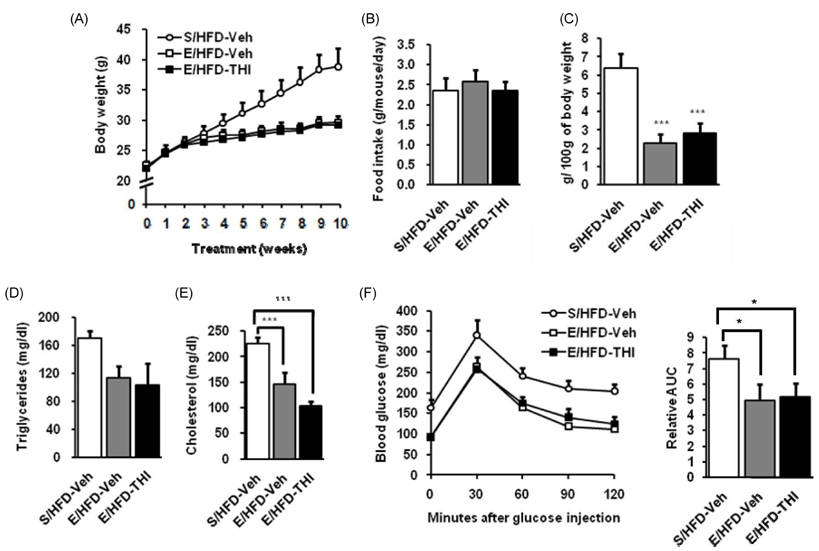
- Target herbal ingredient (THI) is an extract made from two herbs, Scutellariae Radix and Platycodi Radix. It has been developed as a treatment for metabolic diseases such as hyperlipidemia, atherosclerosis, and hypertension. One component of these two herbs has been reported to have anti-inflammatory, anti-hyperlipidemic, and anti-obesity activities. However, there have been no reports about the effects of the mixed extract of these two herbs on metabolic diseases. In this study, we investigated the metabolic effects of THI using a diet-induced obesity (DIO) mouse model. High-fat diet (HFD) mice were orally administered daily with 250 mg/kg of THI. After 10 weeks of treatment, the THI-administered HFD mice showed reduction of body weights and epididymal white adipose tissue weights as well as improved glucose tolerance. In addition, the level of total cholesterol in the serum was markedly reduced. To elucidate the molecular mechanism of the metabolic effects of THI in vitro, 3T3-L1 cells were treated with THI, after which the mRNA levels of adipogenic transcription factors, including C/EBPalpha and PPARgamma, were measured. The results show that the expression of these two transcription factors was down regulated by THI in a dose-dependent manner. We also examined the combinatorial effects of THI and swimming exercise on metabolic status. THI administration simultaneously accompanied by swimming exercise had a synergistic effect on serum cholesterol levels. These findings suggest that THI could be developed as a supplement for improving metabolic status.
Keyword
MeSH Terms
-
3T3-L1 Cells
Adipose Tissue, White
Animals
Atherosclerosis
Body Weight
Cholesterol
Diet, High-Fat
Flavonoids
Glucose
Hyperlipidemias
Hypertension
Metabolic Diseases
Mice
Obesity
PPAR gamma
RNA, Messenger
Scutellaria baicalensis
Swimming
Transcription Factors
Weights and Measures
Cholesterol
Flavonoids
Glucose
PPAR gamma
RNA, Messenger
Transcription Factors
Figure
Reference
-
1. Ahn KS, Noh EJ, Zhao HL, Jung SH, Kang SS, Kim YS. Inhibition of inducible nitric oxide synthase and cyclooxygenase II by Platycodon grandiflorum saponins via suppression of nuclear factor-kappaB activation in RAW 264.7 cells. Life Sci. 2005. 76:2315–2328.
Article2. Choi CY, Kim JY, Kim YS, Chung YC, Hahm KS, Jeong HG. Augmentation of macrophage functions by an aqueous extract isolated from Platycodon grandiflorum. Cancer Lett. 2001. 166:17–25.
Article3. Choi CY, Kim JY, Kim YS, Chung YC, Seo JK, Jeong HG. Aqueous extract isolated from Platycodon grandiflorum elicits the release of nitric oxide and tumor necrosis factor-alpha from murine macrophages. Int Immunopharmacol. 2001. 1:1141–1151.
Article4. Kim YP, Lee EB, Kim SY, Li D, Ban HS, Lim SS, Shin KH, Ohuchi K. Inhibition of prostaglandin E2 production by platycodin D isolated from the root of Platycodon grandiflorum. Planta Med. 2001. 67:362–364.
Article5. Han LK, Zheng YN, Xu BJ, Okuda H, Kimura Y. Saponins from platycodi radix ameliorate high fat diet-induced obesity in mice. J Nutr. 2002. 132:2241–2245.
Article6. Zhao HL, Sim JS, Shim SH, Ha YW, Kang SS, Kim YS. Antiobese and hypolipidemic effects of platycodin saponins in diet-induced obese rats: evidences for lipase inhibition and calorie intake restriction. Int J Obes (Lond). 2005. 29:983–990.
Article7. Lee H, Kang R, Kim YS, Chung SI, Yoon Y. Platycodin D inhibits adipogenesis of 3T3-L1 cells by modulating Kruppel-like factor 2 and peroxisome proliferator-activated receptor gamma. Phytother Res. 2010. 24 Suppl 2. S161–S167.8. Krakauer T, Li BQ, Young HA. The flavonoid baicalin inhibits superantigen-induced inflammatory cytokines and chemokines. FEBS Lett. 2001. 500:52–55.
Article9. Liu LY, Wei EQ, Zhao YM, Chen FX, Wang ML, Zhang WP, Chen Z. Protective effects of baicalin on oxygen/glucose deprivation- and NMDA-induced injuries in rat hippocampal slices. J Pharm Pharmacol. 2005. 57:1019–1026.
Article10. Zhao Y, Li H, Gao Z, Xu H. Effects of dietary baicalin supplementation on iron overload-induced mouse liver oxidative injury. Eur J Pharmacol. 2005. 509:195–200.
Article11. Lee H, Kang R, Hahn Y, Yang Y, Kim SS, Cho SH, Chung SI, Yoon Y. Antiobesity effect of baicalin involves the modulations of proadipogenic and antiadipogenic regulators of the adipogenesis pathway. Phytother Res. 2009. 23:1615–1623.
Article12. Lee H, Bae S, Kim K, Kim W, Chung SI, Yoon Y. Beta-Catenin mediates the anti-adipogenic effect of baicalin. Biochem Biophys Res Commun. 2010. 398:741–746.13. Kasturi R, Joshi VC. Hormonal regulation of stearoyl coenzyme A desaturase activity and lipogenesis during adipose conversion of 3T3-L1 cells. J Biol Chem. 1982. 257:12224–12230.
Article14. Rayalam S, Della-Fera MA, Baile CA. Phytochemicals and regulation of the adipocyte life cycle. J Nutr Biochem. 2008. 19:717–726.
Article15. Hwang JT, Park IJ, Shin JI, Lee YK, Lee SK, Baik HW, Ha J, Park OJ. Genistein, EGCG, and capsaicin inhibit adipocyte differentiation process via activating AMP-activated protein kinase. Biochem Biophys Res Commun. 2005. 338:694–699.
Article16. Bai L, Pang WJ, Yang YJ, Yang GS. Modulation of Sirt1 by resveratrol and nicotinamide alters proliferation and differentiation of pig preadipocytes. Mol Cell Biochem. 2008. 307:129–140.
Article17. Kim SJ, Lee KH, Lee YS, Mun EG, Kwon DY, Cha YS. Transcriptome analysis and promoter sequence studies on early adipogenesis in 3T3-L1 cells. Nutr Res Pract. 2007. 1:19–28.
Article18. Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994. 8:1224–1234.
Article19. Rosen ED, Hsu CH, Wang X, Sakai S, Freeman MW, Gonzalez FJ, Spiegelman BM. C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway. Genes Dev. 2002. 16:22–26.20. Hui KM, Huen MS, Wang HY, Zheng H, Sigel E, Baur R, Ren H, Li ZW, Wong JT, Xue H. Anxiolytic effect of wogonin, a benzodiazepine receptor ligand isolated from Scutellaria baicalensis Georgi. Biochem Pharmacol. 2002. 64:1415–1424.
Article21. Enomoto R, Koshiba C, Suzuki C, Lee E. Wogonin potentiates the antitumor action of etoposide and ameliorates its adverse effects. Cancer Chemother Pharmacol. 2011. 67:1063–1072.
Article22. Nagai T, Yamada H, Otsuka Y. Inhibition of mouse liver sialidase by the root of Scutellaria baicalensis. Planta Med. 1989. 55:27–29.
Article23. Li BQ, Fu T, Gong WH, Dunlop N, Kung H, Yan Y, Kang J, Wang JM. The flavonoid baicalin exhibits anti-inflammatory activity by binding to chemokines. Immunopharmacology. 2000. 49:295–306.
Article24. Lixuan Z, Jingcheng D, Wenqin Y, Jianhua H, Baojun L, Xiaotao F. Baicalin attenuates inflammation by inhibiting NF-kappaB activation in cigarette smoke induced inflammatory models. Pulm Pharmacol Ther. 2010. 23:411–419.
Article25. Du G, Han G, Zhang S, Lin H, Wu X, Wang M, Ji L, Lu L, Yu L, Liang W. Baicalin suppresses lung carcinoma and lung metastasis by SOD mimic and HIF-1alpha inhibition. Eur J Pharmacol. 2010. 630:121–130.
Article26. Kim J, Arias EB, Cartee GD. Effects of gender and prior swim exercise on glucose uptake in isolated skeletal muscles from mice. J Physiol Sci. 2006. 56:305–312.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of a Pholiota adiposa Extract on Fat Mass in Hyperlipidemic Mice
- Sorghum extract exerts an anti-diabetic effect by improving insulin sensitivity via PPAR-gamma in mice fed a high-fat diet
- Comparison of fertility competence in toll-like receptor 4 (TLR4)-knock out male mice fed a high-fat diet
- Anti-obesity effects of Rapha diet(R) preparation in mice fed a high-fat diet
- Effects of Intermittent Fasting on Splenic Galectin-3 Protein Expression in High-fat Diet-fed Mice