Nutr Res Pract.
2012 Aug;6(4):322-327.
Sorghum extract exerts an anti-diabetic effect by improving insulin sensitivity via PPAR-gamma in mice fed a high-fat diet
- Affiliations
-
- 1Department of Food and Nutrition, Hanyang University, 222, Wangsimni-ro, Seongdong-gu, Seoul 133-791, Korea. yongsoon@hanyang.ac.kr
- 2Department of Applied Life Science, Konkuk University, Seoul 143-701, Korea.
Abstract
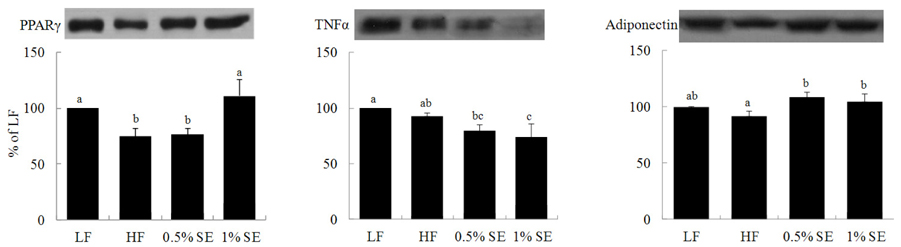
- This study investigated the hypothesis that a sorghum extract exerts anti-diabetic effects through a mechanism that improves insulin sensitivity via peroxisome proliferator-activated receptor gamma (PPAR-gamma) from adipose tissue. Seven C57BL/6 mice were fed an AIN-93M diet with fat consisting of 10% of total energy intake (LF) for 14 weeks, and 21 mice were fed a high-fat AIN diet with 60% of calories derived from fat (HF). From week 8, the HF diet-fed mice were orally administered either saline (HF group), 0.5% (0.5% SE group), or 1% sorghum extract (1% SE group) for 6 weeks (n = 7/group). Perirenal fat content was significantly lower in the 0.5% SE and 1% SE groups than that in the HF mice. Levels of total and low-density lipoprotein cholesterol, triglycerides, glucose, and the area under the curve for glucose were significantly lower in mice administered 0.5% SE and 1% SE than those in HF mice. Serum insulin level was significantly lower in mice administered 1% SE than that in HF mice or those given 0.5% SE. PPAR-gamma expression was significantly higher, whereas the expression of tumor necrosis factor-alpha was significantly lower in mice given 1% SE compared to those in the HF mice. Adiponectin expression was also significantly higher in mice given 0.5% SE and 1% SE than that in the HF mice. These results suggest that the hypoglycemic effect of SE may be related with the regulation of PPAR-gamma-mediated metabolism in this mouse model.
Keyword
MeSH Terms
-
Adiponectin
Adipose Tissue
Animals
Cholesterol
Diet
Diet, High-Fat
Energy Intake
Glucose
Hypoglycemic Agents
Insulin
Insulin Resistance
Lipoproteins
Mice
PPAR gamma
Sorghum
Triglycerides
Tumor Necrosis Factor-alpha
Adiponectin
Cholesterol
Glucose
Hypoglycemic Agents
Insulin
Lipoproteins
PPAR gamma
Triglycerides
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Craig ME, Hattersley A, Donaghue KC. Definition, epidemiology and classification of diabetes in children and adolescents. Pediatr Diabetes. 2009. 10:Suppl 12. 3–12.
Article2. Agius L. New hepatic targets for glycaemic control in diabetes. Best Pract Res Clin Endocrinol Metab. 2007. 21:587–605.
Article3. Hotamisligil GS. The role of TNFalpha and TNF receptors in obesity and insulin resistance. J Intern Med. 1999. 245:621–625.4. Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001. 7:941–946.
Article5. Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, Nagaretani H, Matsuda M, Komuro R, Ouchi N, Kuriyama H, Hotta K, Nakamura T, Shimomura I, Matsuzawa Y. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001. 50:2094–2099.
Article6. Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J Biol Chem. 1995. 270:12953–12956.
Article7. Bouskila M, Pajvani UB, Scherer PE. Adiponectin: a relevant player in PPARgamma-agonist-mediated improvements in hepatic insulin sensitivity? Int J Obes (Lond). 2005. 29:Suppl 1. S17–S23.8. Spranger J, Kroke A, Möhlig M, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF. Adiponectin and protection against type 2 diabetes mellitus. Lancet. 2003. 361:226–228.
Article9. Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995. 95:2409–2415.
Article10. Food and Agriculture Organization of the United Nations [Internet]. FAOSTAT Database. cited 2003 March 25. Rome, Italy: FAO;Available from: http://faostat.fao.org/.11. Kamath VG, Chandrashekar A, Rajini PS. Antiradical properties of sorghum (Sorghum bicolor L. Moench) flour extracts. J Cereal Sci. 2004. 40:283–288.
Article12. Sikwese FE, Duodu KG. Antioxidant effect of a crude phenolic extract from sorghum bran in sunflower oil in the presence of ferric ions. Food Chem. 2007. 104:324–331.
Article13. Park MY, Jang HH, Kim JB, Yoon HN, Lee JY, Lee YM, Kim JH, Park DS. Hog millet (Panicum miliaceum L.)-supplemented diet ameliorates hyperlipidemia and hepatic lipid accumulation in C57BL/6J-ob/ob mice. Nutr Res Pract. 2011. 5:511–519.
Article14. Carr TP, Weller CL, Schlegel VL, Cuppett SL, Guderian DM Jr, Johnson KR. Grain sorghum lipid extract reduces cholesterol absorption and plasma non-HDL cholesterol concentration in hamsters. J Nutr. 2005. 135:2236–2240.
Article15. Hoi JT, Weller CL, Schlegel VL, Cuppett SL, Lee JY, Carr TP. Sorghum distillers dried grain lipid extract increases cholesterol excretion and decreases plasma and liver cholesterol concentration in hamsters. J Funct Foods. 2009. 1:381–386.
Article16. van Rensburg SJ. Epidemiologic and dietary evidence for a specific nutritional predisposition to esophageal cancer. J Natl Cancer Inst. 1981. 67:243–251.17. Chung IM, Kim EH, Yeo MA, Kim SJ, Seo MC, Moon HI. Antidiabetic effects of three Korean sorghum phenolic extracts in normal and streptozotocin-induced diabetic rats. Food Res Int. 2011. 44:127–132.
Article18. Chung IM, Yeo MA, Kim SJ, Kim MJ, Park DS, Moon HI. Antilipidemic activity of organic solvent extract from Sorghum bicolor on rats with diet-induced obesity. Hum Exp Toxicol. 2011. 30:1865–1868.
Article19. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972. 18:499–502.
Article20. Kim JS, Hyun TK, Kim MJ. The inhibitory effects of ethanol extracts from sorghum, foxtail millet and proso millet on α-glucosidase and α-amylase activities. Food Chem. 2011. 124:1647–1651.
Article21. Ruzaidi A, Amin I, Nawalyah AG, Hamid M, Faizul HA. The effect of Malaysian cocoa extract on glucose levels and lipid profiles in diabetic rats. J Ethnopharmacol. 2005. 98:55–60.
Article22. Akase T, Shimada T, Harasawa Y, Akase T, Ikeya Y, Nagai E, Iizuka S, Nakagami G, Iizaka S, Sanada H, Aburada M. Preventive effects of Salacia reticulata on obesity and metabolic disorders in TSOD mice. Evid Based Complement Alternat Med. 2011. 2011:484590.23. Lakshmi KB, Vimala V. Hypoglycemic effect of selected sorghum recipes. Nutr Res. 1996. 16:1651–1658.
Article24. Ray TK, Mansell KM, Knight LC, Malmud LS, Owen OE, Boden G. Long-term effects of dietary fiber on glucose tolerance and gastric emptying in noninsulin-dependent diabetic patients. Am J Clin Nutr. 1983. 37:376–381.
Article25. Lee SH, Chung IM, Cha YS, Park Y. Millet consumption decreased serum concentration of triglyceride and C-reactive protein but not oxidative status in hyperlipidemic rats. Nutr Res. 2010. 30:290–296.
Article26. Chawla A, Schwarz EJ, Dimaculangan DD, Lazar MA. Peroxisome proliferator-activated receptor (PPAR) gamma: adiposepredominant expression and induction early in adipocyte differentiation. Endocrinology. 1994. 135:798–800.
Article27. Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994. 79:1147–1156.
Article28. Willson TM, Brown PJ, Sternbach DD, Henke BR. The PPARs: from orphan receptors to drug discovery. J Med Chem. 2000. 43:527–550.
Article29. Arner P. The adipocyte in insulin resistance: key molecules and the impact of the thiazolidinediones. Trends Endocrinol Metab. 2003. 14:137–145.
Article30. Thamer C, Machann J, Tschritter O, Haap M, Wietek B, Dahl D, Bachmann O, Fritsche A, Jacob S, Stumvoll M, Schick F, Häring HU. Relationship between serum adiponectin concentration and intramyocellular lipid stores in humans. Horm Metab Res. 2002. 34:646–649.
Article31. Bursill CA, Abbey M, Roach PD. A green tea extract lowers plasma cholesterol by inhibiting cholesterol synthesis and upregulating the LDL receptor in the cholesterol-fed rabbit. Atherosclerosis. 2007. 193:86–93.
Article32. Singh DK, Banerjee S, Porter TD. Green and black tea extracts inhibit HMG-CoA reductase and activate AMP kinase to decrease cholesterol synthesis in hepatoma cells. J Nutr Biochem. 2009. 20:816–822.
Article33. Maron DJ, Lu GP, Cai NS, Wu ZG, Li YH, Chen H, Zhu JQ, Jin XJ, Wouters BC, Zhao J. Cholesterol-lowering effect of a theaflavin-enriched green tea extract: a randomized controlled trial. Arch Intern Med. 2003. 163:1448–1453.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of d-alpha-tocopherol supplements on lipid metabolism in a high-fat diet-fed animal model
- Betaine Alleviates Hypertriglycemia and Tau Hyperphosphorylation in db/db Mice
- Effect of a Pholiota adiposa Extract on Fat Mass in Hyperlipidemic Mice
- Sasa borealis extract exerts an antidiabetic effect via activation of the AMP-activated protein kinase
- Anti-obesity effects of hot water extract from Wasabi (Wasabia japonica Matsum.) leaves in mice fed high-fat diets