Nutr Res Pract.
2011 Apr;5(2):107-111.
Quercetin attenuates fasting and postprandial hyperglycemia in animal models of diabetes mellitus
- Affiliations
-
- 1Department of Smart Foods and Drugs, School of Food and Life Science, Inje University, 607 Obang-dong, Gimhae, Gyungnam 621-749, Korea. fdsnkiji@inje.ac.kr
- 2Department of Nutrition, Pusan Paik Hospital, Busan 633-165, Korea.
Abstract
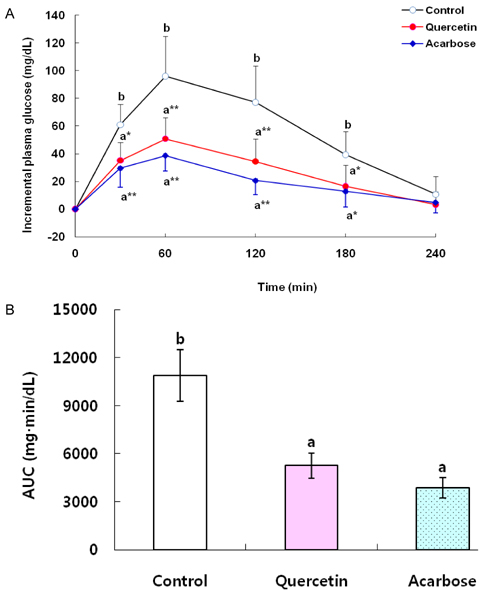
- The objective of this study was to investigate the hypoglycemic effects of quercetin (QE) in animal models of diabetes mellitus (DM). A starch solution (1 g/kg) with and without QE (100 mg/kg) or acarbose (40 mg/kg) was orally administered to streptozotocin (STZ)-induced diabetic rats after an overnight fast. Postprandial plasma glucose levels were measured and incremental areas under the response curve were calculated. To study the effects of chronic feeding of QE, five-week-old db/db mice were fed an AIN-93G diet, a diet containing QE at 0.08%, or a diet containing acarbose at 0.03% for 7 weeks after 1 week of adaptation. Plasma glucose and insulin, blood glycated hemoglobin, and maltase activity of the small intestine were measured. Oral administration of QE (100 mg/kg) or acarbose (40 mg/kg) to STZ-treated rats significantly decreased incremental plasma glucose levels 30-180 min after a single oral dose of starch and the area under the postprandial glucose response, compared with the control group. QE (0.08% of diet) or acarbose (0.03% of diet) offered to db/db mice significantly reduced both plasma glucose and blood glycated hemoglobin compared to controls without significant influence on plasma insulin. Small intestine maltase activities were significantly reduced by consumption of QE or acarbose. Thus, QE could be effective in controlling fasting and postprandial blood glucose levels in animal models of DM.
MeSH Terms
Figure
Reference
-
1. Cheng D. Prevalence, predisposition and prevention of type II diabetes. Nutr Metab (Lond). 2005. 2:29.
Article2. American Diabetes Association (ADA). Summary of revisions for the 2008 clinical practice recommendations. Diabetes Care. 2008. 31:S3–S4.3. Lorenzati B, Zucco C, Miglietta S, Lamberti F, Bruno G. Oral hypoglycemic drugs: Pathophysiological basis of their mechanism of action. Pharmaceuticals. 2010. 3:3005–3020.
Article4. Adewole SO, Caxton-Martins EA, Ojewole JAO. Protective effect of quercetin on the morphology of pancreatic β-cells of streptozotocin-treated diabetic rats. Afr J Tradit Complement Altern Med. 2006. 4:64–74.
Article5. Coskun O, Kanter M, Korkmaz A, Oter S. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and β-cell damage in rat pancreas. Pharmacol Res. 2005. 51:117–123.
Article6. Shetty AK, Rashmi R, Rajan MGR, Sambaiah K, Salimath PV. Antidiabetic influence of quercetin in streptozotocin-induced diabetic rats. Nutr Res. 2004. 24:373–381.
Article7. Ramachandra R, Shetty AK, Salimath PV. Quercetin alleviates activities of intestinal and renal disaccharidases in streptozotocin-induced diabetic rats. Mol Nutr Food Res. 2005. 49:355–360.
Article8. Anjaneyulu M, Chopra K. Quercetin, an anti-oxidant bioflavonoid, attenuates diabetic nephropathy in rats. Clin Exp Pharmacol Physiol. 2004. 31:244–248.
Article9. Ishikawa A, Yamashita H, Hiemori M, Inagaki E, Kimoto M, Okamoto M, Tsuji H, Memon AN, Mohammadio A, Natori Y. Characterization of inhibitors of postprandial hyperglycemia from the leaves of Nerium indicum. J Nutr Sci Vitaminol (Tokyo). 2007. 53:166–173.
Article10. Jo SH, Ka EH, Lee HS, Apostolidis E, Jang HD, Kwon YI. Comparison of antioxidant potential and rat intestinal α-glucosidases inihibitory activities of quercetin, rutin, and isoquercetin. Int J Appl Res Nat Prod. 2009. 2:52–60.11. Standl E, Baumgartl HJ, Füchtenbusch M, Stemplinger J. Effect of acarbose on additional insulin therapy in type 2 diabetic patients with late failure of sulphonylurea therapy. Diabetes Obes Metab. 1999. 1:215–220.
Article12. Abrahamson MJ. Optimal glycemic control in type 2 diabetes mellitus: Fasting and postprandial glucose in context. Arch Intern Med. 2004. 164:486–491.
Article13. Kim YM, Jeong YK, Wang MH, Lee WY, Rhee HI. Inhibitory effect of pine extract on α-glucosidase activity and postprandial hyperglycemia. Nutrition. 2005. 21:756–761.
Article14. Tsujita T, Takaku T. Mechanism of the inhibitory action of chestnut astringent skin extract on carbohydrate absorption. J Nutr Sci Vitaminol (Tokyo). 2008. 54:416–421.
Article15. Akbarzadeh A, Norouzian D, Mehrabi MR, Jamshidi Sh, Farhangi A, Allah Verdi A, Mofidian SMA, Lame Rad B. Induction of diabetes by streptozotocin in rats. Indian J Clin Biochem. 2007. 22:60–64.
Article16. Lee SK, Hwang JY, Song JH, Jo JR, Kim MJ, Kim ME, Kim JI. Inhibitory activity of Euonymus alatus against alpha-glucosidase in vitro and in vivo. Nutr Res Pract. 2007. 1:184–188.17. Raabo E, Terkildsen TC. On the enzymatic determination of blood glucose. Scand J Clin Lab Invest. 1960. 12:402–407.
Article18. Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993. 123:1939–1951.
Article19. Schifreen RS, Hickingbotham JM, Bowers GN Jr. Accuracy, precision, and stability in measurement of hemoglobin A1c by "high-performance" cation-exchange chromatography. Clin Chem. 1980. 26:466–472.
Article20. Morgan CR, Lazarow A. Immunoassay of insulin: Two antibody system. Plasma insulin levels in normal, subdiabetic, and diabetic rats. Diabetes. 1963. 12:115–126.
Article21. Dahlqvist A. Assay of intestinal disaccharidases. Scand J Clin Lab Invest. 1984. 44:169–172.
Article22. Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951. 193:265–275.
Article23. Madar Z. The effect of acarbose and miglitol (BAY-M-1099) on postprandial glucose levels following ingestion of various sources of starch by nondiabetic and streptozotocin-induced diabetic rats. J Nutr. 1989. 119:2023–2029.
Article24. Kang MJ, Kim JH, Choi HN, Kim MJ, Han JH, Lee JH, Kim JI. Hypoglycemic effects of Welsh onion in animal model of diabetes mellitus. Nutr Res Pract. 2010. 4:486–491.
Article25. Ceriello A. Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes. 2005. 54:1–7.26. Ishida H, Takizawa M, Ozawa S, Nakamichi Y, Yamaguchi S, Katsuta H, Tanaka T, Maruyama M, Katahira H, Yoshimoto K, Itagaki E, Nagamatsu S. Pioglitazone improves insulin secretory capacity and prevents the loss of β-cell mass in obese diabetic db/db mice: Possible protection of β cells from oxidative stress. Metabolism. 2004. 53:488–494.
Article27. Srinivasan K, Ramarao P. Animal models in type 2 diabetes research: an overview. Indian J Med Res. 2007. 125:451–472.28. Hummel KP, Coleman DL, Lane PW. The influence of genetic background on expression of mutations at the diabetes locus in the mouse. I. C57BL/KsJ and C57BL/6J strains. Biochem Genet. 1972. 7:1–13.
Article29. Dimitriadis GD, Tessari P, Go VLW, Gerich JE. α-Glucosidase inhibition improves postprandial hyperglycemia and decreases insulin requirements in insulin-dependent diabetes mellitus. Metabolism. 1985. 34:261–265.
Article30. Carrascosa JM, Molero JC, Fermín Y, Martínez C, Andrés A, Satrústegui J. Effects of chronic treatment with acarbose on glucose and lipid metabolism in obese diabetic Wistar rats. Diabetes Obes Metab. 2001. 3:240–248.
Article31. Kannappan S, Anuradha CV. Insulin sensitizing actions of fenugreek seed polyphenols, quercetin & metformin in a rat model. Indian J Med Res. 2009. 129:401–408.32. Juretić D, Bernik Š, Čop L, Hadžija M, Petlevski R, Lukač-Bajalo J. Short-term effect of acarbose on specific intestinal disaccharidase activities and hyperglycaemia in CBA diabetic mice. J Anim Physiol Anim Nutr (Berl). 2003. 87:263–268.
Article33. Lee SM, Bustamante S, Flores C, Bezerra J, Goda T, Koldovský O. Chronic effects of an α-glucosidase inhibitor (Bay o 1248) on intestinal disaccharidase activity in normal and diabetic mice. J Pharmacol Exp Ther. 1987. 240:132–137.34. Liu L, Yu YL, Yang JS, Li Y, Liu YW, Liang Y, Liu XD, Xie L, Wang GJ. Berberine suppresses intestinal disaccharidases with beneficial metabolic effects in diabetic states, evidences from in vivo and in vitro study. Naunyn Schmiedebergs Arch Pharmacol. 2010. 381:371–381.
Article35. Bressler R, Johnson D. New pharmacological approaches to therapy of NIDDM. Diabetes Care. 1992. 15:792–805.
Article36. Theoharides TC, Bielory L. Mast cells and mast cell mediators as targets of dietary supplements. Ann Allergy Asthma Immunol. 2004. 93:S24–S34.
Article