Nutr Res Pract.
2007 Dec;1(4):285-290.
Resveratrol blunts tumor necrosis factor-alpha-induced monocyte adhesion and transmigration
- Affiliations
-
- 1Department of Food and Nutrition and Korean Institute of Nutrition, Hallym University, Chuncheon, Korea. yhkang@hallym.ac.kr
- 2Department of Food and Nutrition, Seoul National University, Seoul, Korea.
Abstract
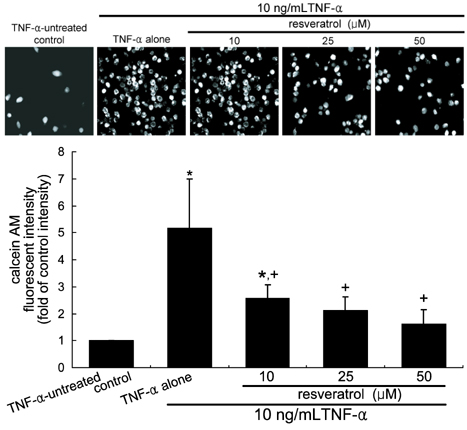
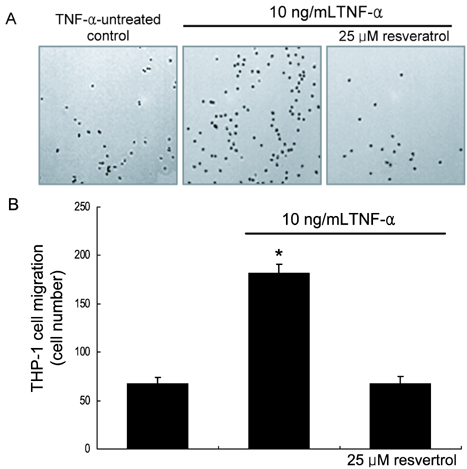
- The leukocyte recruitment and transmigration across the endothelial barrier into the vessel wall are crucial steps in atherosclerosis. Leukocyte trafficking on the endothelium is elicited by induction of endothelial adhesion molecules, and its transmigration is mediated by degradation of basement membrane proteins through enzymatic activity of matrix metalloproteinases (MMP). The current study investigated whether resveratrol, a polyphenol present in grapes and red wine, was capable of inhibiting leukocyte adhesion to tumor necrosis factor (TNF)-alpha-activated endothelium. It was found that resveratrol inhibited the TNF-alpha-activated endothelial expression of vascular cell adhesion molecule-1 in a dose-dependent manner. In addition, resveratrol hampered THP-1 monocyte adhesion to activated endothelial cells. This study further examined whether resveratrol interfered with transendothelial migration of leukocytes. The MMP-2 gelatinolytic activity of endothelial cells was enhanced by TNF-alpha, which was attenuated by an addition of > or =25 micrometer resveratrol. In addition, 25 micrometer resveratrol mitigated the MMP-9 activity of THP-1 cells, followed by a marked inhibition of transendothelial migration. These results demonstrated that resveratrol suppressed monocyte adhesion and migration induced by TNF-alpha through modulating expression of adhesion molecules and gelatinolytic activity of MMP. These findings suggest that dietary resveratrol may be therapeutic agent for inhibiting leukocyte recruitment into the subendothelium during inflammatory atherosclerosis.
Keyword
MeSH Terms
-
Atherosclerosis
Basement Membrane
Endothelial Cells
Endothelium
Leukocytes
Matrix Metalloproteinases
Monocytes*
Necrosis*
Transendothelial and Transepithelial Migration
Tumor Necrosis Factor-alpha
Vascular Cell Adhesion Molecule-1
Vitis
Wine
Matrix Metalloproteinases
Tumor Necrosis Factor-alpha
Vascular Cell Adhesion Molecule-1
Figure
Reference
-
1. Ahn J, Lee H, Kim S, Ha T. Resveratrol inhibits TNF-α-induced changes of adipokines in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2007. 364:972–977.
Article2. Aviram M, Fuhrman B. Wine flavonoids protect against LDLoxidation and atherosclerosis. Ann NY Acad Sci. 2002. 957:146–161.3. Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006. 5:493–506.
Article4. Belleville J. The French paradox: possible involvement of ethanol in the protective effect against cardiovascular diseases. Nutrition. 2002. 18:173–177.
Article5. Berk BC, Abe JI, Min W, Surapisitchat H, Yan C. Endothelial atheroprotective and anti-inflammatory mechanisms. Ann N Y Acad Sci. 2001. 947:93–109.
Article6. Bertelli AA, Giovannini L, Giannessi D, Migliori M, Bernini W, Fregoni M, Bertelli A. Antiplatelet activity of synthetic and natural resveratrol in red wine. Int J Tissue React. 1995. 17:1–3.7. Bradamante S, Barenghi L, Villa A. Cardiovascular protective effects of resveratrol. Cardiovasc Drug Rev. 2004. 22:169–188.
Article8. Choi YJ, Jeong YJ, Lee YJ, Kwon HM, Kang YH. (-) Epigallocatechin gallate and quercetin enhance survival signaling in response to oxidant-induced human endothelial apoptosis. J Nutr. 2005. 135:707–713.
Article9. Chow SE, Hshu YC, Wang JS, Chen JK. Resveratrol attenuates oxLDL-stimulated NADPH oxidase activity and protects endothelial cells from oxidative functional damages. J Appl Physiol. 2007. 102:1520–1527.
Article10. Cullen JP, Morrow D, Jin Y, von Offenberg Sweeney N, Sitzmann JV, Cahill PA, Redmond EM. Resveratrol inhibits expression and binding activity of the monocyte chemotactic protein-1 receptor, CCR2, on THP-1 monocytes. Atherosclerosis. 2007. 195:e125–e133.
Article11. de Lange DW, Verhoef S, Gorter G, Kraaijenhagen RJ, van de Wiel A, Akkerman JW.. Polyphenolic grape extract inhibits platelet activation through PECAM-1: an explanation for the French paradox. Alcohol Clin Exp Res. 2007. 31:1308–1314.
Article12. Fukuhara K, Nagakawa M, Nakanishi I, Ohkubo K, Imai K, Urano S, Fukuzumi S, Ozawa T, Ikota N, Mochizuki M, Miyata N, Okuda H. Structural basis for DNA-cleaving activity of resveratrol in the presence of Cu2+. Bioorg Med Chem. 2006. 14:1437–1443.
Article13. Gagliano N, Moscheni C, Torri C, Magnani I, Bertelli AA, Gioia M. Effect of resveratrol on matrix metalloproteinase-2 (MMP-2) and Secreted Protein Acidic and Rich in Cysteine (SPARC) on human cultured glioblastoma cells. Biomed Pharmacother. 2005. 59:359–364.
Article14. Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994. 94:2493–2503.
Article15. Hao HD, He LR. Mechanisms of cardiovascular protection by resveratrol. J Med Food. 2004. 7:290–298.
Article16. Hu J, Huang Y, Xiong M, Luo S, Chen Y, Li Y. The effects of natural flavonoids on lipoxygenase-mediated oxidation of compounds with a benzene ring structure-a new possible mechanism of flavonoid anti-chemical carcinogenesis and other toxicities. Int J Toxicol. 2006. 25:295–301.
Article17. Katiyar SK. Matrix metalloproteinases in cancer metastasis: molecular targets for prostate cancer prevention by green tea polyphenols and grape seed proanthocyanidins. Endocr Metab Immune Disord Drug Targets. 2006. 6:17–24.
Article18. Krasinski K, Spyridopoulos I, Kearney M, Losordo DW. In vivo blockade of tumor necrosis factor-alpha accelerates functional endothelial recovery after balloon angioplasty. Circulation. 2000. 104:1754–1756.
Article19. Middleton E Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications forinflammation heart disease and cancer. Pharmacol Rev. 2000. 52:673–751.20. Nagaoka I, Hirota S. Increased expression of matrix metalloproteinase-9 in neutrophils in glycogen-induced peritoneal inflammation of guinea pigs. Inflamm Res. 2000. 49:55–62.
Article21. Nakashima Y, Raines EW, Plump AS, Breslow JL, Ross R. Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the Apo-E-deficient mouse. Arterioscler Thromb Vasc Biol. 1998. 18:842–851.
Article22. Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001. 74:418–425.
Article23. Pober JS, Cotran RS. The role of endothelial cells in inflammation. Transplantation. 1990. 50:537–544.
Article24. Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993. 362:801–809.
Article25. Saarialho-Kere UK, Welgus HG, Parks WC. Distinct mechanisms regulate interstitial collagenase and 92-kDa gelatinase expression in human monocytic-like cells exposed to bacterial endotoxin. J Biol Chem. 1993. 268:17354–17361.
Article26. Schonbeck U, Mach F, Libby P. CD154 (CD40 ligand). Int J Biochem Cell Biol. 2000. 32:687–693.
Article27. Shankar S, Singh G, Srivastava RK. Chemoprevention by resveratrol: molecular mechanisms and therapeutic potential. Front Biosci. 2007. 12:4839–4854.
Article28. Stoclet JC, Chataigneau T, Ndiaye M, Oak MH, El Bedoui J, Chataigneau M, Schini-Kerth VB. Vascular protection by dietary polyphenols. Eur J Pharmacol. 2004. 500:299–313.
Article29. Teixeira S, Siquet C, Alves C, Boal I, Marques MP, Borges F, Lima JL, Reis S. Structure-property studies on the antioxidant activity of flavonoids present in diet. Free Radic Biol Med. 2005. 39:1099–1108.
Article30. Woo JH, Lim JH, Kim YH, Suh SI, Min DS, Chang JS, Lee YH, Park JW, Kwon TK. Resveratrol inhibits phorbol myristate acetate-induced matrix metalloproteinase-9 expression by inhibiting JNK and PKC delta signal transduction. Oncogene. 2004. 23:1845–1853.
Article31. Wung BS, Hsu MC, Wu CC, Hsieh CW. Resveratrol suppresses IL-6-induced ICAM-1 gene expression in endothelial cells: effects on the inhibition of STAT3 phosphorylation. Life Sci. 2005. 78:389–397.
Article32. Zhu Z, Klironomos G, Vachereau A, Neirinck L, Goodman DW. Determination of trans-resveratrol in human plasma by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 1999. 724:389–392.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- NADPH Oxidase and Mitochondrial ROS are Involved in the TNF-alpha-induced Vascular Cell Adhesion Molecule-1 and Monocyte Adhesion in Cultured Endothelial Cells
- TNF-alpha-induced up-regulation of intercellular adhesion molecule-1 is regulated by a Rac-ROS-dependent cascade in human airway epithelial cells
- A standardized bamboo leaf extract inhibits monocyte adhesion to endothelial cells by modulating vascular cell adhesion protein-1
- The Inflammatory Response and Cardiac Repair After Myocardial Infarction
- Elevated Tumor Necrosis Factor-alpha in Stable Angina Pectoris