Korean Circ J.
2009 Oct;39(10):393-398. 10.4070/kcj.2009.39.10.393.
The Inflammatory Response and Cardiac Repair After Myocardial Infarction
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, College of Medicine, Dongguk University, Gyeongju, Korea. ptca@dongguk.ac.kr
- KMID: 2028918
- DOI: http://doi.org/10.4070/kcj.2009.39.10.393
Abstract
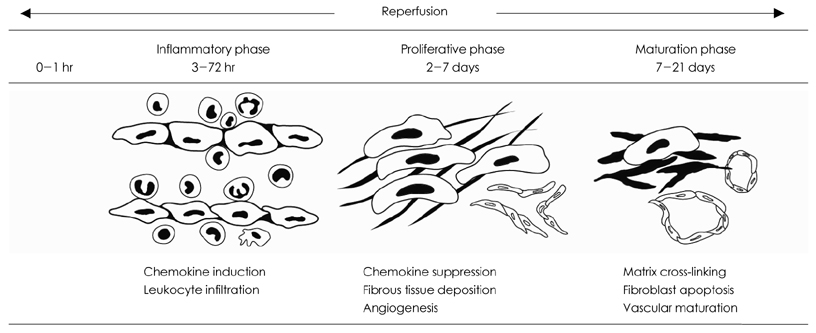
- One of the most important therapeutic targets of current cardiology practice is to determine optimal strategies for the minimization of myocardial necrosis and optimization of cardiac repair following an acute myocardial infarction. Myocardial necrosis after acute myocardial infarction induces complement activation and free radical generation, triggering a cytokine cascade initiated by tumor necrosis factor-alpha (TNF-alpha) release. When reperfusion of the infarcted area is initiated, intense inflammation follows. Chemokines, cytokines and the complement system play an important role in recruiting neutrophils in the ischemic and reperfused myocardium. Cytokines promote adhesive interactions between leukocytes and endothelial cells, resulting in transmigration of inflammatory cells into the site of injury. The recruited neutrophils have potent cytotoxic effects through the release of proteolytic enzymes, and they interact with adhesion molecules on cardiomyocytes. In spite of the potential injury, reperfusion enhances cardiac repair; this may be related to the inflammatory response. Monocyte chemoattractant protein (MCP)-1 is upregulated in reperfused myocardium and can induce monocyte recruitment in the infarcted area. Monocyte subsets play a role in phagocytosis of dead cardiomyocytes and in granulation tissue formation. In addition, the transforming growth factor (TGF)-beta plays a crucial role in cardiac repair by suppressing inflammation. Resolution of inflammatory infiltration, containment of inflammation and the reparative response affecting the infarcted area are essential for optimal infarct healing. Here, we review the current literature on the inflammatory response and cardiac repair after myocardial infarction.
MeSH Terms
-
Adhesives
Cardiology
Chemokines
Complement Activation
Complement System Proteins
Containment of Biohazards
Cytokines
Endothelial Cells
Granulation Tissue
Inflammation
Leukocytes
Monocytes
Myocardial Infarction
Myocardium
Myocytes, Cardiac
Necrosis
Neutrophils
Peptide Hydrolases
Phagocytosis
Reperfusion
Reperfusion Injury
Transforming Growth Factors
Tumor Necrosis Factor-alpha
Ventricular Remodeling
Adhesives
Chemokines
Complement System Proteins
Cytokines
Peptide Hydrolases
Transforming Growth Factors
Tumor Necrosis Factor-alpha
Figure
Cited by 1 articles
-
Therapeutic Hypothermia for Cardioprotection in Acute Myocardial Infarction
In Sook Kang, Ikeno Fumiaki, Wook Bum Pyun
Yonsei Med J. 2016;57(2):291-297. doi: 10.3349/ymj.2016.57.2.291.
Reference
-
1. Jennings RB, Murry CE, Steenbergen C Jr, Reimer KA. Development of cell injury in sustained acute ischemia. Circulation. 1990. 82:3 Suppl. II2–II12.2. Deten A, Volz HC, Briest W, Zimmer HG. Cardiac cytokine expression is upregulated in the acute phase after myocardial in farction: experimental studies in rats. Cardiovasc Res. 2002. 55:329–340.3. Irwin M, Mak S, Mann DL, et al. Tissue expression and immunolocalization of tumour necrosis factor-alpha in post infarction-dysfunctional myocardium. Circulation. 1999. 99:1492–1498.4. Birdsall HH, Green DM, Trial J, et al. Complement C5a, TGF-beta 1, and MCP-1, in sequence, induce migration of monocytes into ischemic canine myocardium within the first one to five hours after reperfusion. Circulation. 1997. 95:684–692.5. Dewald O, Zymek P, Winkelmann K, et al. CCL2/monocyte chemoattractant protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res. 2005. 96:881–889.6. Bujak M, Frangogiannis NG. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res. 2007. 74:184–195.7. Hill JH, Ward PA. The phlogistic role of C3 leukotactic fragments in myocardial infarcts of rats. J Exp Med. 1971. 133:885–900.8. Pinckard RN, Olson MS, Giclas PC, Terry R, Boyer JT, O'Rourke RA. Consumption of classical complement components by heart subcellular membranes in vitro and in patients after acute myocardial infarction. J Clin Invest. 1975. 56:740–750.9. Yasojima K, Schwab C, McGeer EG, McGeer PL. Human heart generates complement proteins that are upregulated and activated after myocardial infarction. Circ Res. 1998. 83:860–869.10. Dreyer WJ, Michael LH, Nguyen T, et al. Kinetics of C5a release in cardiac lymph of dogs experiencing coronary artery ischemia-reperfusion injury. Circ Res. 1992. 71:1518–1524.11. Weisman HF, Bartow T, Leppo MK, et al. Soluble human complement receptor type 1: in vivo inhibitor of complement suppressing post-ischemic myocardial inflammation and necrosis. Science. 1990. 249:146–151.12. Granger CB, Mahaffey KW, Weaver WD, et al. Pexelizumab, an anti-C5 complement antibody, as adjunctive therapy to primary percutaneous coronary intervention in acute myocardial infarction: the complement inhibition inmyocardial infarction treated with angioplasty (COMMA) trial. Circulation. 2003. 108:1184–1190.13. Mahaffey KW, Granger CB, Nicolau JC, et al. Effect of pexelizumab, an anti-C5 complement antibody, as adjunctive therapy to fibrinolysis in acute myocardial infarction: the COMPlement inhibition in myocardial infarction treated with thromboLYtics (COMPLY) trial. Circulation. 2003. 108:1176–1183.14. Armstrong PW, Granger CB, Adams PX, et al. Pexelizumab for acute ST-elevation myocardial infarction in patients undergoing primary percutaneous coronary intervention: a randomized controlled trial. JAMA. 2007. 297:43–51.15. Meldrum DR, Dinarello CA, Cleveland JC Jr, et al. Hydrogen peroxide induces tumor necrosis factor alphamediated cardiac injury by a p38 mitogen activated protein kinasedependent mechanisms. Surgery. 1998. 124:291–296. discussion 297.16. Shingu M, Nobunaga M. Chemotactic activity generated in human serum from the fifth component of complement by hydrogen peroxide. Am J Pathol. 1984. 117:201–206.17. Akgur FM, Brown MF, Zibari GB, et al. Role of superoxide in hemorrhagic shock-induced P-selectin expression. Am J Physiol Heart Circ Physiol. 2000. 279:H791–H797.18. Lakshminarayanan V, Beno DW, Costa RH, Roebuck KA. Differential regulation of interleukin-8 and intercellular adhesion molecule-1 by H2O2 and tumor necrosis factor-alpha in endothelial and epithelial cells. J Biol Chem. 1997. 272:32910–32918.19. Sellak H, Franzini E, Hakim J, Pasquier C. Reactive oxygen species rapidly increase endothelial ICAM-1 ability to bind neutrophils without detectable upregulation. Blood. 1994. 83:2669–2677.20. Jolly SR, Kane WJ, Bailie MB, Abrams GD, Lucchesi BR. Canine myocardial reperfusion injury: its reduction by the combined administration of superoxide dismutase and catalase. Circ Res. 1984. 54:277–285.21. Uraizee A, Reimer KA, Murry CE, Jennings RB. Failure of superoxide dismutase to limit size of myocardial infarction after 40 minutes of ischemia and 4 days of reperfusion in dogs. Circulation. 1987. 75:1237–1248.22. Gallagher KP, Buda AJ, Pace D, Gerren RA, Shlafer M. Failure of superoxide dismutase and catalase to alter size of infarction in conscious dogs after 3 hours of occlusion followed by reperfusion. Circulation. 1986. 73:1065–1076.23. Murohara Y, Yui Y, Hattori R, Kawai C. Effects of superoxide dismutase on reperfusion arrhythmias and left ventricular function in patients undergoing thrombolysis for anterior wall acute myocardial infarction. Am J Cardiol. 1991. 67:765–767.24. Flaherty JT, Pitt B, Gruber JW, et al. Recombinant human superoxide dismutase (h-SOD) fails to improve recovery of ventricular function in patients undergoing coronary angioplasty for acute myocardial infarction. Circulation. 1994. 89:1982–1991.25. Siwik DA, Chang DL, Coluci WS. Interleukin-1 beta and tumor necrosis factor-alpha decrease collagen synthesis and increase matrix metalloproteinase activity in cardiac fibroblasts in vitro. Circ Res. 2000. 86:1259–1265.26. Kumar AG, Ballantyne CM, Michael LH, et al. Induction of monocyte chemoattractant protein-1 in the small veins of the ischemic and reperfused canine myocardium. Circulation. 1997. 95:693–700.27. Frangogiannis NG. Chemokines in ischemia and reperfusion. Thromb Haemost. 2007. 97:738–747.28. Tarzami ST, Cheng R, Miao W, Kitsis RN, Berman JW. Chemokine expression in myocardial ischemia: MIP-2 dependent MCP-1 expression protects cardiomyocytes from cell death. J Mol Cell Cardiol. 2002. 34:209–221.29. Gwechenberger M, Mendoza LH, Youker KA, et al. Cardiac myocytes produce interleukin-6 in culture and in viable border zone of reperfused infarctions. Circulation. 1999. 99:546–551.30. Frangogiannis NG, Youker KA, Entman ML. The role of the neutrophil in myocardial ischemia and reperfusion. EXS. 1996. 76:263–284.31. Romson JL, Hook BG, Kunkel SL, Abrams GD, Schork MA, Lucchesi BR. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation. 1983. 67:1016–1023.32. Jordan JE, Zhao ZQ, Vinten-Johansen J. The role of neutrophils in myocardial ischemia-reperfusion injury. Cardiovasc Res. 1999. 43:860–878.33. Jaeschke H, Smith CW. Mechanisms of neutrophil-induced parenchymal cell injury. J Leukoc Biol. 1997. 61:647–653.34. Palazzo AJ, Jones SP, Anderson DC, Granger DN, Lefer DJ. Coronary endothelial P-selectin in pathogenesis of myocardial ischemia-reperfusion injury. Am J Physiol. 1998. 275:H1865–H1872.35. Jones SP, Girod WG, Granger DN, Palazzo AJ, Lefer DJ. Reperfusion injury is not affected by blockade of P-selectin in the diabetic mouse heart. Am J Physiol. 1999. 277:H763–H769.36. Birnbaum Y, Patterson M, Kloner RA. The effect of CY1503, a sialyl Lewisx analog blocker of the selectin adhesion molecules, on infarct size and "noreflow" in the rabbit model of acute myocardial infarction/reperfusion. J Mol Cell Cardiol. 1997. 29:2013–2025.37. Frangogiannis NG. The mechanistic basis of infarct healing. Antioxid Redox Signal. 2006. 8:1907–1939.38. Nathan C. Points of control in inflammation. Nature. 2002. 420:846–852.39. Thompson NL, Bazoberry F, Speir EH, et al. Transforming growth factor beta-1 in acute myocardial infarction in rats. Growth Factors. 1988. 1:91–99.40. Dean RG, Balding LC, Candido R, et al. Connective tissue growth factor and cardiac fibrosis after myocardial infarction. J Histochem Cytochem. 2005. 53:1245–1256.41. Bassols A, Massague J. Transforming growth factor beta regulates the expression and structure of extracellular matrix chondroitin/dermatan sulfate proteoglycans. J Biol Chem. 1988. 263:3039–3045.42. Lefer AM, Tsao P, Aoki N, Palladino MA Jr. Mediation of cardioprotection by transforming growth factor-beta. Science. 1990. 249:61–64.43. Frantz S, Hu K, Adammek A, et al. Transforming growth factor beta inhibition increases mortality and left ventricular dilatation after myocardial infarction. Basic Res Cardiol. 2008. 103:485–492.44. Ikeuchi M, Tsutsui H, Shiomi T, et al. Inhibition of TGF-beta signaling exacerbates early cardiac dysfunction but prevents late remodeling after infarction. Cardiovasc Res. 2004. 64:526–535.45. Okada H, Takemura G, Kosai K, et al. Postinfarction gene therapy against transforming growth factor-beta signal modulates infarct tissue dynamics and attenuates left ventricular remodeling and heart failure. Circulation. 2005. 111:2430–2437.46. Youn TJ, Kim HS, Oh BH. Ventricular remodeling and transforming growth factor-beta 1 mRNA expression after nontransmural myocardial infarction in rats: effects of angiotensin converting enzyme inhibition and angiotensin II type 1 receptor blockade. Basic Res Cardiol. 1999. 94:246–253.47. Frangogiannis NG. The immune system and cardiac repair. Pharmacol Res. 2008. 58:88–111.48. Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996. 87:2095–2147.49. Bujak M, Dobaczewski M, Chatila K, et al. Interleukin-1 receptor type I signaling critically regulates infarct healing and cardiac remodeling. Am J Pathol. 2008. 173:57–67.50. de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991. 174:1209–1220.51. Moore KW, deWaal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001. 19:683–765.52. Lacraz S, Nicod LP, Chicheportiche R, Welgus HG, Dayer JM. IL-10 inhibits metalloproteinase and stimulates TIMP-1 production in human mononuclear phagocytes. J Clin Invest. 1995. 96:2304–2310.53. Zymek P, Nah DY, Bujak M, et al. Interleukin-10 is not a critical regulator of infarct healing and left ventricular remodeling. Cardiovasc Res. 2007. 74:313–322.54. Mikecz K, Brennan FR, Kim JH, Glant TT. Anti-CD44 treatment abrogates tissue edema and leukocyte infiltration in murine arthritis. Nat Med. 1995. 1:558–563.55. DeGrendele HC, Estess P, Siegelman MH. Requirement for CD44 in activated T cell extravasation into an inflammatory site. Science. 1997. 278:672–675.56. Teder P, Vandivier RW, Jiang D, et al. Resolution of lung inflammation by CD44. Science. 2002. 296:155–158.57. Huebener P, Abou-Khamis T, Zymek P, et al. CD44 is critically involved in infarct healing by regulating the inflammatory and fibrotic response. J Immunol. 2008. 180:2625–2633.58. Lawler J. Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. J Cell Mol Med. 2002. 6:1–12.59. Frangogiannis NG, Ren G, Dewald O, et al. The critical role of endogenous thrombospondin (TSP)-1 in preventing expansion of healing myocardial infarcts. Circulation. 2005. 111:2935–2942.60. Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003. 113:685–700.61. Hao J, Ju H, Zhao S, Junail A, Scammell-La Fleur T, Dixon IM. Elevation of expression of Smads 2, 3, and 4, decorin and TGF-beta in the chronic phase of myocardial infarct scar healing. J Mol Cell Cardiol. 1999. 31:667–678.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- KLF9 deficiency protects the heart from inflammatory injury triggered by myocardial infarction
- A Case of Early Developed Left Ventricular Free Wall Rupture Followed by Acute Inferior Myocardial Infarction
- Aconitine Intoxication Misdiagnosed as Acute Myocardial Infarction
- Cardiac arrest due to an unexpected acute myocardial infarction during head and neck surgery: A case report
- Myocardial Infarction in a Patient with Myocardial Bridge and Pheochromocytoma: A case report