Nutr Res Pract.
2013 Feb;7(1):9-14.
A standardized bamboo leaf extract inhibits monocyte adhesion to endothelial cells by modulating vascular cell adhesion protein-1
- Affiliations
-
- 1Infectious Signaling Network Research Center and Research Institute for Medical Sciences, Department of Physiology, School of Medicine, Chungnam National University, 6 Munhwa-dong, Jung-gu, Daejeon 301-747, Korea. bhjeon@cnu.ac.kr
- 2Unigen Inc., Cheonan, Chungnam 330-863, Korea.
- 3Life Science Research Institute, Univera Inc., Seoul 133-120, Korea.
- 4Basic Herbal Medicine Research Group, Korea Institute of Oriental Medicine, Daejeon 305-811, Korea.
Abstract
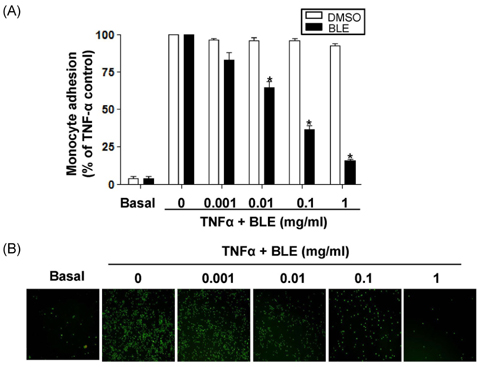
- Bamboo leaves (Phyllostachys pubescens Mazel ex J. Houz (Poacea)) have a long history of food and medical applications in Asia, including Japan and Korea. They have been used as a traditional medicine for centuries. We investigated the mechanism of anti-inflammatory activity of a bamboo leaf extract (BLE) on tumor necrosis factor-alpha (TNF-alpha)-induced monocyte adhesion in human umbilical vein endothelial cells (HUVECs). Exposure of HUVECs to BLE did not inhibit cell viability or cause morphological changes at concentrations ranging from 1 microg/ml to 1 mg/ml. Treatment with 0.1 mg/ml BLE caused 63% inhibition of monocyte adhesion in TNF-alpha-activated HUVECs, which was associated with 38.4% suppression of vascular cell adhesion molecule-1 expression. Furthermore, TNF-alpha-induced reactive oxygen species generation was decreased to 47.9% in BLE treated TNF-alpha-activated HUVECs. BLE (0.05 mg/ml) also caused about 50% inhibition of interleukin-6 secretion from lipopolysaccharide-stimulated monocyte. The results indicate that BLE may be clinically useful as an anti-inflammatory or anti-oxidant for human cardiovascular disease including atherosclerosis.
Keyword
MeSH Terms
-
Asia
Atherosclerosis
Cardiovascular Diseases
Cell Adhesion
Cell Survival
Endothelial Cells
Human Umbilical Vein Endothelial Cells
Humans
Interleukin-6
Japan
Korea
Medicine, Traditional
Monocytes
Reactive Oxygen Species
Tumor Necrosis Factor-alpha
Vascular Cell Adhesion Molecule-1
Interleukin-6
Reactive Oxygen Species
Tumor Necrosis Factor-alpha
Vascular Cell Adhesion Molecule-1
Figure
Reference
-
1. Flavahan NA. Atherosclerosis or lipoprotein-induced endothelial dysfunction. Potential mechanisms underlying reduction in EDRF/nitric oxide activity. Circulation. 1992. 85:1927–1938.
Article2. Harrison DG. Endothelial dysfunction in atherosclerosis. Basic Res Cardiol. 1994. 89:Suppl 1. 87–102.
Article3. Calabresi PA, Prat A, Biernacki K, Rollins J, Antel JP. T lymphocytes conditioned with Interferon beta induce membrane and soluble VCAM on human brain endothelial cells. J Neuroimmunol. 2001. 115:161–167.
Article4. James WG, Bullard DC, Hickey MJ. Critical role of the alpha 4 integrin/VCAM-1 pathway in cerebral leukocyte trafficking in lupus-prone MRL/fas(lpr) mice. J Immunol. 2003. 170:520–527.
Article5. Ley K, Huo Y. VCAM-1 is critical in atherosclerosis. J Clin Invest. 2001. 107:1209–1210.
Article6. Eriksson EE, Xie X, Werr J, Thoren P, Lindbom L. Importance of primary capture and L-selectin-dependent secondary capture in leukocyte accumulation in inflammation and atherosclerosis in vivo. J Exp Med. 2001. 194:205–218.
Article7. McEver RP. Adhesive interactions of leukocytes, platelets, and the vessel wall during hemostasis and inflammation. Thromb Haemost. 2001. 86:746–756.
Article8. Steeber DA, Tedder TF. Adhesion molecule cascades direct lymphocyte recirculation and leukocyte migration during inflammation. Immunol Res. 2000. 22:299–317.
Article9. Wackers FJ, Soufer R, Zaret BL. Braunwald E, Zipes D, Libby P, editors. Nuclear cardiology. Heart Disease: A Textbook of Cardiovascular Medicine. 2001. 6th ed. Philadelphia: Saunders;288–290.10. Campbell JH, Efendy JL, Smith NJ, Campbell GR. Molecular basis by which garlic suppresses atherosclerosis. J Nutr. 2001. 131:1006S–1009S.
Article11. Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993. 342:1007–1011.
Article12. Knekt P, Jarvinen R, Reunanen A, Maatela J. Flavonoid intake and coronary mortality in Finland: a cohort study. BMJ. 1996. 312:478–481.
Article13. Grassi D, Aggio A, Onori L, Croce G, Tiberti S, Ferri C, Ferri L, Desideri G. Tea, flavonoids, and nitric oxide-mediated vascular reactivity. J Nutr. 2008. 138:1554S–1560S.
Article14. Shah AJ, Gilani AH. Aqueous-methanolic extract of sweet flag (Acorus calamus) possesses cardiac depressant and endothelialderived hyperpolarizing factor-mediated coronary vasodilator effects. J Nat Med. 2012. 66:119–126.
Article15. Kweon MH, Hwang HJ, Sung HC. Identification and antioxidant activity of novel chlorogenic acid derivatives from bamboo (Phyllostachys edulis). J Agric Food Chem. 2001. 49:4646–4655.
Article16. Park HS, Lim JH, Kim HJ, Choi HJ, Lee IS. Antioxidant flavone glycosides from the leaves of Sasa borealis. Arch Pharm Res. 2007. 30:161–166.
Article17. Seki T, Maeda H. Cancer preventive effect of Kumaizasa bamboo leaf extracts administered prior to carcinogenesis or cancer inoculation. Anticancer Res. 2010. 30:111–118.18. Kim CS, Son SJ, Kim EK, Kim SN, Yoo DG, Kim HS, Ryoo SW, Lee SD, Irani K, Jeon BH. Apurinic/apyrimidinic endonuclease1/redox factor-1 inhibits monocyte adhesion in endothelial cells. Cardiovasc Res. 2006. 69:520–526.
Article19. Cho EJ, Park MS, Kim SS, Kang G, Choi S, Lee YR, Chang SJ, Lee KH, Lee SD, Park JB, Jeon BH. Vasorelaxing activity of Ulmus davidiana ethanol extracts in rats: activation of endothelial nitric oxide synthase. Korean J Physiol Pharmacol. 2011. 15:339–344.
Article20. Kim SN, Son SC, Lee SM, Kim CS, Yoo DG, Lee SK, Hur GM, Park JB, Jeon BH. Midazolam inhibits proinflammatory mediators in the lipopolysaccharide-activated macrophage. Anesthesiology. 2006. 105:105–110.
Article21. Weber KS, von Hundelshausen P, Clark-Lewis I, Weber PC, Weber C. Differential immobilization and hierarchical involvement of chemokines in monocyte arrest and transmigration on inflamed endothelium in shear flow. Eur J Immunol. 1999. 29:700–712.
Article22. Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther. 2006. 8:Suppl 2. S3.23. Cao LH, Lee YJ, Kang DG, Kim JS, Lee HS. Effect of Zanthoxylum schinifolium on TNF-alpha-induced vascular inflammation in human umbilical vein endothelial cells. Vascul Pharmacol. 2009. 50:200–207.
Article24. Roy S, Sen CK, Packer L. Determination of cell-cell adhesion in response to oxidants and antioxidants. Methods Enzymol. 1999. 300:395–401.
Article25. Bevilacqua MP, Pober JS, Wheeler ME, Cotran RS, Gimbrone MA Jr. Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest. 1985. 76:2003–2011.
Article26. Faruqi RM, DiCorleto PE. Mechanisms of monocyte recruitment and accumulation. Br Heart J. 1993. 69:S19–S29.
Article27. Lum H, Roebuck KA. Oxidant stress and endothelial cell dysfunction. Am J Physiol Cell Physiol. 2001. 280:C719–C741.
Article28. Chen YH, Lin SJ, Ku HH, Shiao MS, Lin FY, Chen JW, Chen YL. Salvianolic acid B attenuates VCAM-1 and ICAM-1 expression in TNF-alpha-treated human aortic endothelial cells. J Cell Biochem. 2001. 82:512–521.
Article29. Hung CF, Huang TF, Chen BH, Shieh JM, Wu PH, Wu WB. Lycopene inhibits TNF-alpha-induced endothelial ICAM-1 expression and monocyte-endothelial adhesion. Eur J Pharmacol. 2008. 586:275–282.
Article30. Murphy N, Grimsditch DC, Vidgeon-Hart M, Groot PH, Overend P, Benson GM, Graham A. Dietary antioxidants decrease serum soluble adhesion molecule (sVCAM-1, sICAM-1) but not chemokine (JE/MCP-1, KC) concentrations, and reduce atherosclerosis in C57BL but not apoE*3 Leiden mice fed an atherogenic diet. Dis Markers. 2005. 21:181–190.
Article31. Lakota K, Mrak-Poljsak K, Rozman B, Sodin-Semrl S. Increased responsiveness of human coronary artery endothelial cells in inflammation and coagulation. Mediators Inflamm. 2009. 2009:146872.
Article32. Islam KN, Devaraj S, Jialal I. alpha-Tocopherol enrichment of monocytes decreases agonist-induced adhesion to human endothelial cells. Circulation. 1998. 98:2255–2261.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Protective Effect of Allium tuberosum Extract on Vascular Inflammation in Tumor Necrosis Factor-α-induced Human Vascular Endothelial Cells
- Allicin Reduces Adhesion Molecules and NO Production Induced by gamma irradiation in Human Endothelial Cells
- NADPH Oxidase and Mitochondrial ROS are Involved in the TNF-alpha-induced Vascular Cell Adhesion Molecule-1 and Monocyte Adhesion in Cultured Endothelial Cells
- Resveratrol blunts tumor necrosis factor-alpha-induced monocyte adhesion and transmigration
- Activated platelets induce secretion of interleukin-1beta, monocyte chemotactic protein-1, and macrophage inflammatory protein-1alpha and surface expression of intercellular adhesion molecule-1 on cultured endothelial cells