Lab Anim Res.
2016 Jun;32(2):99-104. 10.5625/lar.2016.32.2.99.
Anti-Helicobacter pylori activity of crude N-acetylneuraminic acid isolated from glycomacropeptide of whey
- Affiliations
-
- 1Laboratory Animal Resource Center, Daegu Gyeongbuk Institute of Science & Technology (DGIST), Daegu, Korea.
- 2Laboratory Animal Medicine and Brain Korea 21 PLUS Project, College of Veterinary Medicine, Chonnam National University, Gwangju, Korea. jonpark@jnu.ac.kr
- 3Department of Biochemistry, College of Medicine, Konyang University, Daejeon, Korea.
- 4Lifetree Biotechnology Institute, Lifetree Bitotech Co. Ltd., Gyeonggi, Korea.
- KMID: 2309083
- DOI: http://doi.org/10.5625/lar.2016.32.2.99
Abstract
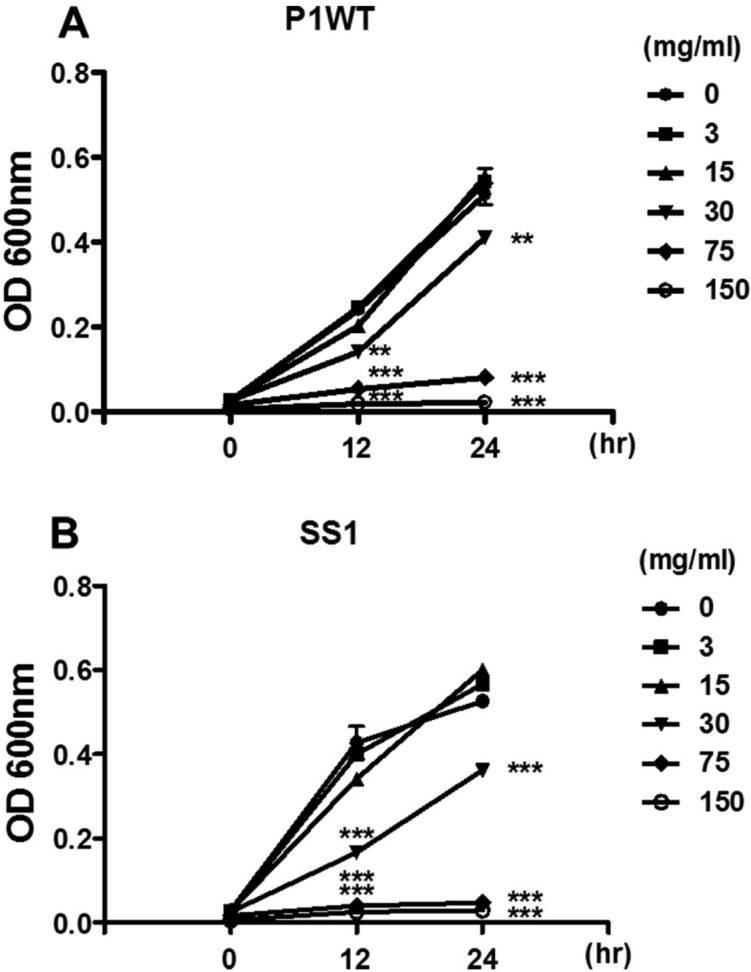
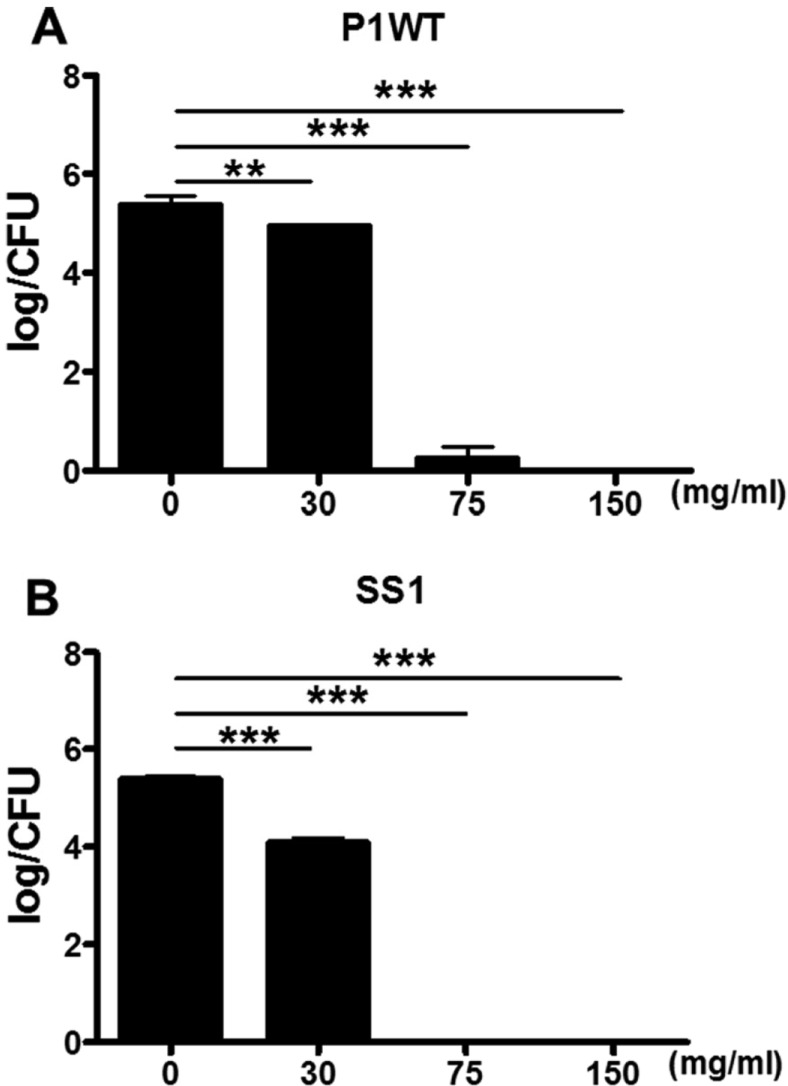
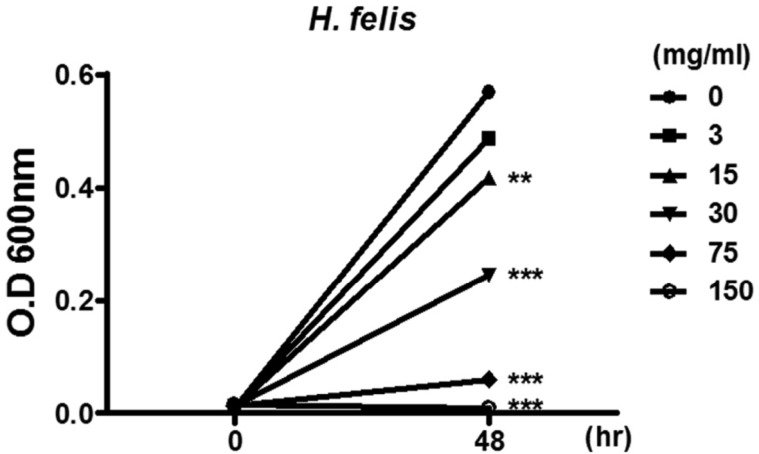
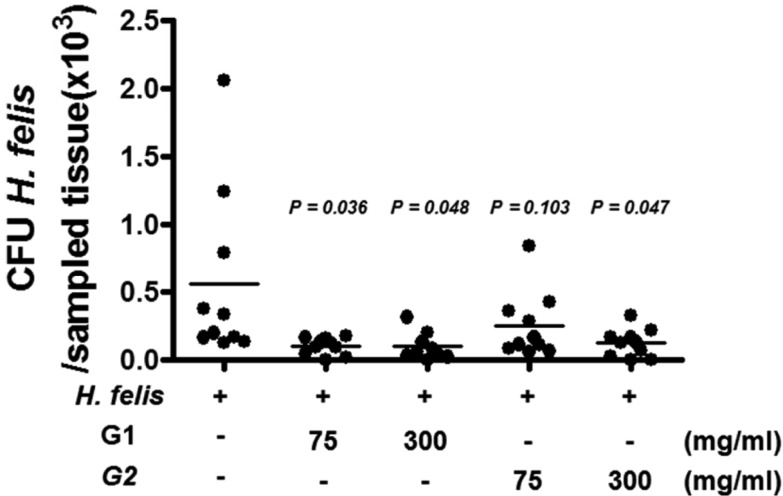
- Helicobacter pylori colonizes the gastric mucosa of about half of the world's population, causing chronic gastritis and gastric cancer. An increasing emergence of antibiotic-resistant H. pylori arouses demand on alternative non-antibiotic-based therapies. In this study, we freshly prepared crude N-acetylneuraminic acid obtained from glycomacropeptide (G-NANA) of whey through a neuraminidase-mediated reaction and evaluated its antibacterial ability against H. pylori and H. felis. Overnight cultures of the H. pylori were diluted with fresh media and different concentrations (1-150 mg/mL) of crude G-NANA were added directly to the culture tube. Bacterial growth was evaluated by measuring the optical density of the culture medium and the number of viable bacteria was determined by a direct count of the colony forming units (CFU) on agar plates. For the in vivo study, mice were orally infected with 100 µL (5×108 cfu/mL) of H. felis four times at a day's interval, accompanied by a daily administration of crude G-NANA or vehicle. A day after the last infection, the mice were daily administered the crude G-NANA (0, 75, and 300 mg/mL) for 10 days and euthanized. Their stomachs were collected and bacterial colonization was determined by quantitative real-time PCR. Crude G-NANA inhibited H. pylori's growth and reduced the number of viable bacteria in a dose-dependent manner. Furthermore, crude G-NANA inhibited bacterial colonization in the mice. These results showed that crude G-NANA has antibacterial activity against Helicobacter and demonstrated its therapeutic potential for the prevention of chronic gastritis and gastric carcinogenesis induced by Helicobacter infection in humans.
MeSH Terms
Figure
Reference
-
1. Huang JQ, Sridhar S, Chen Y, Hunt RH. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology. 1998; 114(6):1169–1179. PMID: 9609753.2. Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001; 345(11):784–789. PMID: 11556297.3. Perez-Perez GI, Rothenbacher D, Brenner H. Epidemiology of Helicobacter pylori infection. Helicobacter. 2004; 9(Suppl 1):1–6. PMID: 15347299.4. Borén T, Falk P, Roth KA, Larson G, Normark S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science. 1993; 262(5141):1892–1895. PMID: 8018146.5. Mobley HLT, Mendz MG, Hazell SL. Helicobacter; physiology and genetics. Washington, D.C: ASM press;2001. p. 381–417.6. Backert S, Selbach M. Role of type IV secretion in Helicobacter pylori pathogenesis. Cell Microbiol. 2008; 10(8):1573–1581. PMID: 18410539.7. Cover TL. The vacuolating cytotoxin of Helicobacter pylori. Mol Microbiol. 1996; 20(2):241–246. PMID: 8733223.8. Sayi A, Kohler E, Hitzler I, Arnold I, Schwendener R, Rehrauer H, Müller A. The CD4+ T cell-mediated IFN-gamma response to Helicobacter infection is essential for clearance and determines gastric cancer risk. J Immunol. 2009; 182(11):7085–7101. PMID: 19454706.9. Akhiani AA, Pappo J, Kabok Z, Schön K, Gao W, Franzén LE, Lycke N. Protection against Helicobacter pylori infection following immunization is IL-12-dependent and mediated by Th1 cells. J Immunol. 2002; 169(12):6977–6984. PMID: 12471132.10. Beswick EJ, Pinchuk IV, Earley RB, Schmitt DA, Reyes VE. Role of gastric epithelial cell-derived transforming growth factor beta in reduced CD4+ T cell proliferation and development of regulatory T cells during Helicobacter pylori infection. Infect Immun. 2011; 79(7):2737–2745. PMID: 21482686.11. O'Connor A, Vaira D, Gisbert JP, O'Morain C. Treatment of Helicobacter pylori infection 2014. Helicobacter. 2014; 19:38–45. PMID: 25167944.12. Wang B, Brand-Miller J. The role and potential of sialic acid in human nutrition. Eur J Clin Nutr. 2003; 57(11):1351–1369. PMID: 14576748.
Article13. Tram TH, Brand Miller JC, McNeil Y, McVeagh P. Sialic acid content of infant saliva: comparison of breast fed with formula fed infants. Arch Dis Child. 1997; 77(4):315–318. PMID: 9389234.
Article14. Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993; 3(2):97–130. PMID: 8490246.
Article15. Parkkinen J, Finne J, Achtman M, Väisänen V, Korhonen TK. Escherichia coli strains binding neuraminyl alpha 2-3 galactosides. Biochem Biophys Res Commun. 1983; 111(2):456–461. PMID: 6340671.16. Idota T, Kawakami H, Murakami Y, Sugawara M. Inhibition of cholera toxin by human milk fractions and sialyllactose. Biosci Biotechnol Biochem. 1995; 59(3):417–419. PMID: 7766178.
Article17. Viala J, Chaput C, Boneca IG, Cardona A, Girardin SE, Moran AP, Athman R, Mémet S, Huerre MR, Coyle AJ, DiStefano PS, Sansonetti PJ, Labigne A, Bertin J, Philpott DJ, Ferrero RL. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat Immunol. 2004; 5(11):1166–1174. PMID: 15489856.18. Kwok T, Zabler D, Urman S, Rohde M, Hartig R, Wessler S, Misselwitz R, Berger J, Sewald N, König W, Backert S. Helicobacter exploits integrin for type IV secretion and kinase activation. Nature. 2007; 449(7164):862–866. PMID: 17943123.
Article19. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25(4):402–408. PMID: 11846609.20. Campbell J. High-throughput assessment of bacterial growth inhibition by optical density measurements. Curr Protoc Chem Biol. 2010; 2(4):195–208. PMID: 23839976.
Article21. Mohammadi M, Redline R, Nedrud J, Czinn S. Role of the host in pathogenesis of Helicobacter-associated gastritis: H. felis infection of inbred and congenic mouse strains. Infect Immun. 1996; 64(1):238–245. PMID: 8557346.
Article22. Flach CF, Svensson N, Blomquist M, Ekman A, Raghavan S, Holmgren J. A truncated form of HpaA is a promising antigen for use in a vaccine against Helicobacter pylori. Vaccine. 2011; 29(6):1235–1241. PMID: 21147129.23. Malfertheiner P1, Schultze V, Rosenkranz B, Kaufmann SH, Ulrichs T, Novicki D, Norelli F, Contorni M, Peppoloni S, Berti D, Tornese D, Ganju J, Palla E, Rappuoli R, Scharschmidt BF, Del Giudice G. Safety and immunogenicity of an intramuscular Helicobacter pylori vaccine in noninfected volunteers: a phase I study. Gastroenterology. 2008; 135(3):787–795. PMID: 18619971.24. Stoicov C, Saffari R, Houghton J. Green tea inhibits Helicobacter growth in vivo and in vitro. Int J Antimicrob Agents. 2009; 33(5):473–478. PMID: 19157800.25. Matsubara S, Shibata H, Ishikawa F, Yokokura T, Takahashi M, Sugimura T, Wakabayashi K. Suppression of Helicobacter pylori-induced gastritis by green tea extract in Mongolian gerbils. Biochem Biophys Res Commun. 2003; 310(3):715–719. PMID: 14550260.26. Tombola F, Campello S, De Luca L, Ruggiero P, Del Giudice G, Papini E, Zoratti M. Plant polyphenols inhibit VacA, a toxin secreted by the gastric pathogen Helicobacter pylori. FEBS Lett. 2003; 543(1-3):184–189. PMID: 12753930.27. Sivam GP. Protection against Helicobacter pylori and other bacterial infections by garlic. J Nutr. 2001; 131(3s):1106S–1108S. PMID: 11238826.28. Fahey JW, Haristoy X, Dolan PM, Kensler TW, Scholtus I, Stephenson KK, Talalay P, Lozniewski A. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc Natl Acad Sci U S A. 2002; 99(11):7610–7615. PMID: 12032331.29. Mahady GB, Pendland SL, Chadwick LR. Resveratrol and red wine extracts inhibit the growth of CagA+ strains of Helicobacter pylori in vitro. Am J Gastroenterol. 2003; 98(6):1440–1441. PMID: 12818294.30. Sgouras D, Maragkoudakis P, Petraki K, Martinez-Gonzalez B, Eriotou E, Michopoulos S, Kalantzopoulos G, Tsakalidou E, Mentis A. In vitro and in vivo inhibition of Helicobacter pylori by Lactobacillus casei strain Shirota. Appl Environ Microbiol. 2004; 70(1):518–526. PMID: 14711683.31. Lorca GL, Wadström T, Valdez GF, Ljungh A. Lactobacillus acidophilus autolysins inhibit Helicobacter pylori in vitro. Curr Microbiol. 2001; 42(1):39–44. PMID: 11116395.32. Pinchuk IV, Bressollier P, Verneuil B, Fenet B, Sorokulova IB, Mégraud F, Urdaci MC. In vitro anti-Helicobacter pylori activity of the probiotic strain Bacillus subtilis 3 is due to secretion of antibiotics. Antimicrob Agents Chemother. 2001; 45(11):3156–3161. PMID: 11600371.33. Johnson-Henry KC, Mitchell DJ, Avitzur Y, Galindo-Mata E, Jones NL, Sherman PM. Probiotics reduce bacterial colonization and gastric inflammation in H. pylori-infected mice. Dig Dis Sci. 2004; 49(7-8):1095–1102. PMID: 15387328.
Article34. Simon PM, Goode PL, Mobasseri A, Zopf D. Inhibition of Helicobacter pylori binding to gastrointestinal epithelial cells by sialic acid-containing oligosaccharides. Infect Immun. 1997; 65(2):750–757. PMID: 9009338.35. Mysore JV, Wigginton T, Simon PM, Zopf D, Heman-Ackah LM, Dubois A. Treatment of Helicobacter pylori infection in rhesus monkeys using a novel antiadhesion compound. Gastroenterology. 1999; 117(6):1316–1325. PMID: 10579973.36. Yang JC, Shun CT, Chien CT, Wang TH. Effective prevention and treatment of Helicobacter pylori infection using a combination of catechins and sialic acid in AGS cells and BALB/c mice. J Nutr. 2008; 138(11):2084–2090. PMID: 18936202.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-bacterial effects of enzymatically-isolated sialic acid from glycomacropeptide in a Helicobacter pylori-infected murine model
- Anti-Helicobacter pylori Activity of Compounds Isolated from Fraxinus mandshurica Bark
- Study on the antimicrobial activities of herbal extracts against Helicobacter pylori
- A New Effective Therapy for Helicobacter pylori Eradication Using a Potassium-Competitive Acid Blocker
- Anti-Helicobacter pylori Compounds from Polygonum cuspidatum