Korean J Urol.
2006 Feb;47(2):195-200. 10.4111/kju.2006.47.2.195.
The Effects of Selective Cyclooxygenase-2 Inhibitor and Prostaglandin E2 Receptor Agonists on the Endothelin Axis of Prostate Cancer Cells
- Affiliations
-
- 1Department of Urology, College of Medicine, Chung-Ang University, Seoul, Korea. kthlmk@hanafos.com
- KMID: 2294201
- DOI: http://doi.org/10.4111/kju.2006.47.2.195
Abstract
-
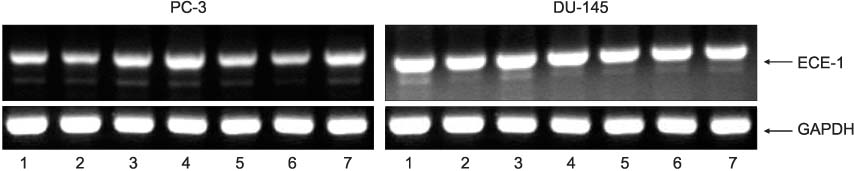
PURPOSE: The enhanced expression of the cyclooxygenase-2 (COX-2), prostaglandin E2 receptor (EPs) and endothelin-1 (ET-1) axis is known to play a significant role in the development and progression of several malignancies. To date, little work has been done to investigate the relationships between the COX-2, EPs and ET-1 axis in prostate cancer (PC) cells. The aim of this study is to investigate the expression of preproET-1 (PPET-1), ET-1 receptor A (ET(A)R), and endothelin converting enzyme-1 (ECE-1) in the PC cell lines and to evaluate the effects of COX-2 and EPs on the expression of PPET-1, ET(A)R, and ECE-1.
MATERIALS AND METHODS
Two PC cell lines, PC-3 and DU-145 cells were used for this study. By performing reverse transcription polymerase chain reaction (RT-PCR), the mRNA expressions of PPET-1, ET(A)R and ECE-1 were detected, and then the mRNA expressions of PPET-1, ET(A)R and ECE-1 were detected after being treating the cells with selective COX-2 inhibitor (NS-398), or EP2 (butaprost) and EP4 (misoprostol), which are both agonist of 10(-10), 10(-8) and 10(-6)M.
RESULTS
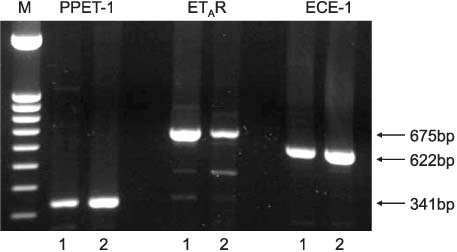
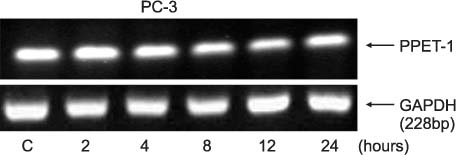
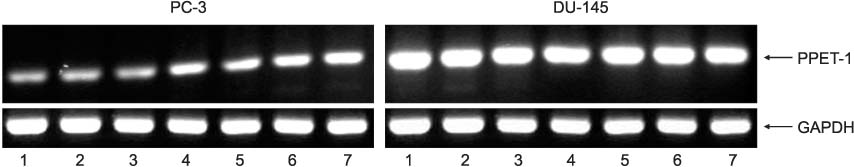
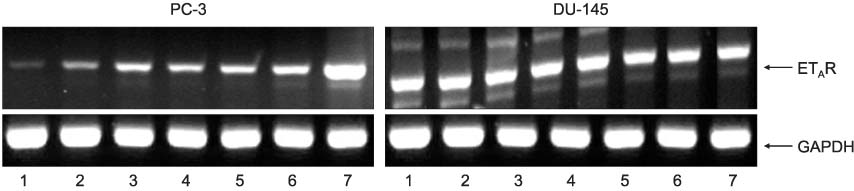
PPET-1, ET(A)R and ECE-1 mRNA were expressed in both cell lines. After NS-398 treatment, only the PPET-1 mRNA expression was decreased at 4, 8 and 12 hours in the PC-3 cells. EP2 and EP4 agonist induced an increase for the PPET-1, ET(A)R and ECE-1 mRNA expressions, compared with the NS-398 treated group (control), in the PC-3 cells.
CONCLUSIONS
ET-1/ET(A)R and ECE-1, whose expressions are increased by EP2 and EP4, may play key roles in the development and progression of PC via COX-2. A combination treatment with selective inhibitors for COX-2, EPs and ET(A)R would be novel approach to prostate cancer therapy.
Keyword
MeSH Terms
Figure
Reference
-
1. Korea Statistical Information System. National Statistical Office. Republic of Korea. Available from: URL: http://kosis.nso.go.kr.2. Lara PN Jr, Meyers FJ. Treatment options in androgen-independent prostate cancer. Cancer Invest. 1999. 17:137–144.3. Masferrer JL, Zweifel BS, Manning PT, Hauser SD, Leahy KM, Smith WG, et al. Selective inhibition of inducible cyclooxygenase-2 in vivo is antiinflammatory and non-ulcerogenic. Proc Natl Acad Sci USA. 1994. 91:3228–3232.4. Chaudry AA, Wahle KW, McClinton S, Moffat LE. Arachidonic acid metabolism in benign and malignant prostatic tissue in vitro: effects of fatty acids and cyclooxygenase inhibitors. Int J Cancer. 1994. 57:176–180.5. Trevisani A, Ferretti E, Capuzzo A, Tomasi V. Elevated levels of prostaglandin E2 in Yoshida hepatoma and the inhibition of tumour growth by non-steroidal anti-inflammatory drugs. Br J Cancer. 1980. 41:341–347.6. Iwasaki Y, Iwasaki J, Freake HC. Growth inhibition of human breast cancer cells induced by calcitonin. Biochem Biophys Res Commun. 1983. 110:235–242.7. Rudland PS, Twiston-Davies AC, Tsao SW. Rat mammary preadipocytes in culture produce a trophic agent for mammary epithelia-prostaglandin E2. J Cell Physiol. 1984. 120:364–376.8. Bandyopadhyay GK, Imagawa W, Wallace D, Nandi S. Linoleate metabolites enhance the in vitro proliferative response of mouse mammary epithelial cells to epidermal growth factor. J Biol Chem. 1987. 262:2750–2756.9. Kim TH, Kim YS, Myoung SC, Lee JH, Won EH. Prostaglandin E receptor II and IV increase the expression of matrix metalloproteinase-7 in PC (prostate cancer)-3 cells. Korean J Urol. 2004. 45:478–484.10. Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988. 332:411–415.11. Masaki T. The endothelin family: an overview. J Cardiovasc Pharmacol. 2000. 35:4 Suppl 2. S3–S5.12. Nelson J, Bagnato A, Battistini B, Nisen P. The endothelin axis: emerging role in cancer. Nat Rev Cancer. 2003. 3:110–116.13. Bagnato A, Catt KJ. Endothelin as autocrine regulators of tumor cell growth. Trends Endocrinol Metab. 1998. 9:378–383.14. Bagnato A, Natali PG. Endothelin receptors as novel targets in tumor therapy. J Transl Med. 2004. 2:16.15. Nelson JB, Hedican SP, George DJ, Reddi AH, Piantadosi S, Eisenberger MA, et al. Identification of endothelin-1 in the pathophysiology of metastatic adenocarcinoma of the prostate. Nat Med. 1995. 1:944–949.16. Grant ES, Brown T, Roach A, Williams BC, Habib FK. In vitro expression of endothelin-1 (ET-1) and ETA and ETB ET receptors by the prostatic epithelium and stroma. J Clin Endocrinol Metab. 1997. 82:508–513.17. Usmani BA, Harden B, Maitland NJ, Turner AJ. Differential expression of neutral endopeptidase-24.11 (neprilysin) and endothelin converting enzyme in human prostate cancer cell lines. Clin Sci. 2002. 103:Suppl 48. S314–S317.18. Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA. 1997. 94:3336–3340.19. Sonoshita M, Takaku K, Sasaki N, Sugimoto Y, Ushikubi F, Narumiya S, et al. Acceleration of intestinal polyposis through prostaglandin receptor EP2 in ApcΔ716 knockout mice. Nat Med. 2001. 7:1048–1051.20. Naraba H, Yokoyama C, Tago N, Murakami M, Kudo I, Fueki M, et al. Transcriptional regulation of the membrane-associated prostaglandin E2 synthase gene. Essential role of the transcription factor Egr-1. J Biol Chem. 2002. 277:28601–28608.21. Yoshimatsu K, Golijanin D, Paty PB, Soslow RA, Jacobsson PJ, DeLellis RA, et al. Inducible microsomal prostaglandin E synthase is overexpressed in colorectal adenomas and cancer. Clin Cancer Res. 2001. 7:3971–3976.22. Pratt PF, Bokemeyer D, Foschi M, Sorokin A, Dunn MJ. Alterations in subcellular localization of p38 MAPK potentiates endothelin-stimulated COX-2 expression in glomerular mesangial cells. J Biol Chem. 2003. 278:51928–51936.23. Spinella F, Rosanò L, Di Castro V, Nicotra MR, Natali PG, Bagnato A. Inhibition of cyclooxygenase-1 and cyclooxygenase-2 expression by targeting the endothelin A receptor in human ovarian carcinoma cells. Clin Cancer Res. 2004. 10:4670–4679.24. Spinella F, Rosanò L, Di Castro V, Natali PG, Bagnato A. Endothelin-1-induced prostaglandin E2-EP2, EP4-signaling regulates vascular endothelial growth factor production and ovarian carcinoma cell invasion. J Biol Chem. 2004. 279:46700–46705.25. Tran J, Master Z, Yu JL, Rak J, Dumont DJ, Kerbel RS. A role for survivin in chemoresistance of endothelial cells mediated by VEGF. Proc Natl Acad Sci USA. 2002. 99:4349–4354.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prostagladin E Receptor II and IV Increase the Expression of Martrix Metalloproteinase-7 in PC (Prostate Cancer)-3 Cells
- The Prostaglandin E Receptor Agonists Increase Granulocyte Macrophage Colony-Stimulating Factor in Prostate Cancer Cells
- Prostaglandin E2 receptors as therapeutic targets in renal fibrosis
- Endoperoxides and Thromboxane A2 in Porcine Coronary Arteries with Regenerated Endothelium
- Melittin-induced Nociceptive Responses are Alleviated by Cyclooxygenase-1 Inhibitor