Korean J Urol.
2006 Mar;47(3):310-315. 10.4111/kju.2006.47.3.310.
The Effect of Cyclooxygenase-2 Inhibitor on the Gene Expression Profile of N-butyl-N-(4-hydroxybutyl) nitrosamine-induced Rat Urinary Bladder Cancer
- Affiliations
-
- 1Department of Urology, and the Urological Science Institute, Brain Korea 21 Project for Medical Science, Yonsei University College of Medicine, Korea. sjhong346@yumc.yonsei.ac.kr
- 2Department of Urology, Hanyang University College of Medicine, Seoul, Korea.
- KMID: 2294180
- DOI: http://doi.org/10.4111/kju.2006.47.3.310
Abstract
-
PURPOSE: Cyclooxygenase (COX)-2 plays an important role in promoting cancer cell proliferation and angiogenesis in human bladder cancer. In this study, we investigated the antitumor or antiangiogenic effects of selective COX-2 inhibitor on N-butyl-N-(4-hydroxybutyl)nitrosamine (BBN)-induced rat bladder tumorigenesis.
MATERIALS AND METHODS
Forty male Fischer 344 rats (control) were given only 0.05% BBN, while 40 rats (experimental) were administered 1,500mg/ kg celecoxib once daily and this treatment started from 1 week before their BBN treatment. Ten rats from the control groups and the experimental groups were then sacrificed at 4, 12, 16 and 24 weeks after BBN treatment. We observed all the bladders macroscopically as well as microscopically, and we measured the COX-2 expression in the bladder tissues. Utilizing a cDNA microarray, we analyzed the significant differences of gene expression between the 12 week-control group and the 12 week-experimental group.
RESULTS
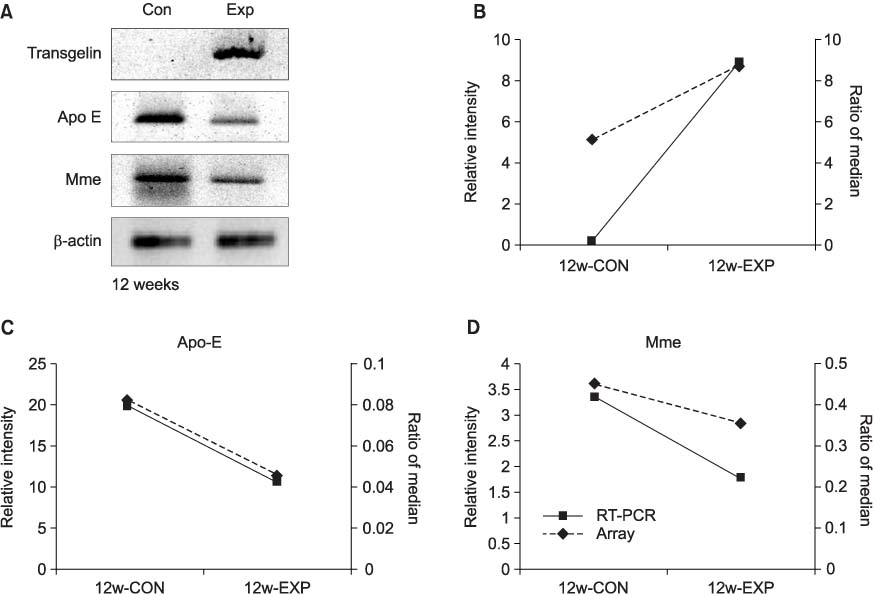
The incidence of tumor was lower in the experimental group than in the control group from week 12 to week 24. The COX-2 expressions were more significantly decreased via the BBN induction (p<0.05) in the experimental groups than in the control groups after 4 weeks. For the 12 week-experimental group, there were 15 genes altered by the administration of selective COX-2 inhibitor, and the selective COX-2 inhibitor especially regulated transgelin, membrane metallo endopeptidase and apolipoprotein E of these 15 genes to prevent the incidence of bladder tumor.
CONCLUSIONS
Selective COX-2 inhibitor has an inhibitory effect on BBN-induced rat bladder tumorigenesis. In the pre-neoplastic phase, selective COX-2 inhibitor regulates transgelin, membrane metallo endopeptidase and apolipoprotein E to prevent the incidence of bladder tumor.
MeSH Terms
-
Angiogenesis Inducing Agents
Animals
Apolipoproteins
Butylhydroxybutylnitrosamine
Carcinogenesis
Cell Proliferation
Cyclooxygenase 2*
Gene Expression*
Humans
Incidence
Male
Neprilysin
Oligonucleotide Array Sequence Analysis
Prostaglandin-Endoperoxide Synthases
Rats*
Transcriptome*
Urinary Bladder Neoplasms*
Urinary Bladder*
Celecoxib
Angiogenesis Inducing Agents
Apolipoproteins
Butylhydroxybutylnitrosamine
Cyclooxygenase 2
Neprilysin
Prostaglandin-Endoperoxide Synthases
Figure
Reference
-
1. Pruthi RS, Derksen E, Gaston K. Cyclooxygenase-2 as a potential target in the prevention and treatment of genitourinary tumors: a review. J Urol. 2003. 169:2352–2359.2. Li G, Yang T, Yan J. Cyclooxygenase-2 increased the angiogenic and metastatic potential of tumor cells. Biochem Biophys Res Commun. 2002. 299:886–890.3. Prescott SM, Fitzpatrick FA. Cyclooxygenase-2 and carcinogenesis. Biochim Biophys Acta. 2000. 1470:M69–M78.4. Ricchi P, Zarrilli R, Di Palma A, Acquaviva AM. Nonsteroidal anti-inflammatory drugs in colorectal cancer: from prevention to therapy. Br J Cancer. 2003. 88:803–807.5. Shirahama T. Cyclooxygenase-2 expression is up-regulated in transitional cell carcinoma and its preneoplastic lesions in the human urinary bladder. Clin Cancer Res. 2000. 6:2424–2430.6. Ristimaki A, Nieminen O, Saukkonen K, Hotakainen K, Nordling S, Haglund C. Expression of cyclooxygenase-2 in human transitional cell carcinoma of the urinary bladder. Am J Pathol. 2001. 158:849–853.7. Kim SI, Kwon SM, Kim YS, Hong SJ. Association of cyclooxygenase-2 expression with prognosis of stage T1 grade 3 bladder cancer. Urology. 2002. 60:816–821.8. Shirahama T, Sakakura C. Overexpression of cyclooxygenase-2 in squamous cell carcinoma of the urinary bladder. Clin Cancer Res. 2001. 7:558–561.9. Friedrich MG, Toma MI, Petri S, Huland H. Cyclooxygenase-2 promotes angiogenesis in pTa/T1 urothelial bladder carcinoma but does not predict recurrence. BJU Int. 2003. 92:389–392.10. Hurst CD, Fiegler H, Carr P, Williams S, Carter NP, Knowles MA. High-resolution analysis of genomic copy number alterations in bladder cancer by microarray-based comparative genomic hybridization. Oncogene. 2004. 23:2250–2263.11. Sanchez-Carbayo M, Cordon-Cardo C. Applications of array technology: identification of molecular targets in bladder cancer. Br J Cancer. 2003. 89:2172–2177.12. Ariel I, Ayesh S, Gofrit O, Ayesh B, Abdul-Ghani R, Pizov G, et al. Gene expression in the bladder carcinoma rat model. Mol Carcinog. 2004. 41:69–76.13. Grubbs CJ, Lubet RA, Koki AT, Leahy KM, Masferrer JL, Steele VE, et al. Celecoxib inhibits N-butyl-N-(4-hydroxybutyl)-nitrosamine-induced urinary bladder cancers in male B6D2F1 and female Fischer-344 rats. Cancer Res. 2000. 60:5599–5602.14. Takei S, Iseda T, Yokoyama M. Inhibitory effect of clotrimazole on angiogenesis associated with bladder epithelium proliferation in rats. Int J Urol. 2003. 10:78–85.15. Osawa S, Terashima Y, Kimura G, Akimoto M. Antitumour effects of the angiogenesis inhibitor AGM-1470 on rat urinary bladder tumours induced by N-butyl-N-(4-hydroxybutyl) nitrosamine. BJU Int. 1999. 83:123–128.16. Kwon SM, Oh HY, Kim SI, Hong SJ. Cyclooxygenase-2 inhibitor delayed the tumorigenesis of N-butyl-N-(4-hydroxybutyl) nitrosamine-induced rat urinary bladder cancer model. Korean J Urol. 2004. 45:578–584.17. Kim JH, Shim JS, Lee SK, Kim KW, Rha SY, Chung HC, et al. Microarray-based analysis of anti-angiogenic activity of demethoxycurcumin on human umbilical vein endothelial cells: crucial involvement of the down-regulation of matrix metalloproteinase. Jpn J Cancer Res. 2002. 93:1378–1385.18. Yang SH, Kim JS, Oh TJ, Kim MS, Lee SW, Woo SK, et al. Genome-scale analysis of resveratrol-induced gene expression profile in human ovarian cancer cells using a cDNA microarray. Int J Oncol. 2003. 22:741–750.19. Seo MY, Rha SY, Yang SH, Kim SC, Lee GY, Park CH, et al. The pattern of gene copy number changes in bilateral breast cancer surveyed by cDNA microarray-based comparative genomic hybridization. Int J Mol Med. 2004. 13:17–24.20. Shintani N, Tomimoto S, Hashimoto H, Kawaguchi C, Baba A. Functional roles of the neuropeptide PACAP in brain and pancreas. Life Sci. 2003. 74:337–343.21. Shields JM, Rogers-Graham K, Der CJ. Loss of transgelin in breast and colon tumors and in RIE-1 cells by Ras deregulation of gene expression through Raf-independent pathways. J Biol Chem. 2002. 277:9790–9799.22. Freedland SJ, Seligson DB, Liu AY, Pantuck AJ, Paik SH, Horvath S, et al. Loss of CD10 (neutral endopeptidase) is a frequent and early event in human prostate cancer. Prostate. 2003. 55:71–80.23. Koiso K, Akaza H, Ohtani M, Miyanaga N, Aoyagi K. A new tumor marker for bladder cancer. Int J Urol. 1994. 1:33–36.24. Venanzoni MC, Giunta S, Muraro GB, Storari L, Crescini C, Mazzucchelli R, et al. Apolipoprotein E expression in localized prostate cancers. Int J Oncol. 2003. 22:779–786.25. Li LS, Kim H, Rhee H, Kim SH, Shin DH, Chung KY, et al. Proteomic analysis distinguishes basaloid carcinoma as a distinct subtype of nonsmall cell lung carcinoma. Proteomics. 2004. 4:3394–3400.26. DeSouza L, Diehl G, Rodrigues MJ, Guo J, Romaschin AD, Colgan TJ, et al. Search for cancer markers from endometrial tissues using differentially labeled tags iTRAQ and clCAT with multidimensional liquid chromatography and tandem mass spectrometry. J Proteome Res. 2005. 4:377–386.27. Perna AG, Smith MJ, Krishnan B, Reed JA. CD10 is expressed in cutaneous clear cell lesions of different histogenesis. J Cutan Pathol. 2005. 32:348–351.28. Galetto R, Albajar M, Polanco JI, Zakin MM, Rodriguez-Rey JC. Identification of a peroxisome-proliferator-activated-receptor response element in the apolipoprotein E gene control region. Biochem J. 2001. 357:521–527.29. McGarvey TW, Nguyen TB, Malkowicz SB. An interaction between apolipoprotein E and TERE1 with a possible association with bladder tumor formation. J Cell Biochem. 2005. 95:419–428.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Experimental Study on Hyperplasia of Urinary Bladder Epithelium Induced by N-Butyl-N-(4-Hydroxybutyl) Nitrosamine in Rats
- Immunohistochemical Study with Carcinoembryonic Antigen in Experimental Bladder Tumor Induced by Administration of N-butyl-N-(4-hydroxybutyl)-nitrosamine
- Effect of intraperitoneal injection of single chemotherapeutic agent on rat bladder carcinogenesis induced by N-butyl-N-(4-hydroxybutyl) nitrosamine
- Expression of Survivin, HSP90, Bcl-2 and Bax Proteins in N-butyl-N-(4-hydroxybutyl)nitrosamine-induced Rat Bladder Carcinogenesis
- Effect of Intraperitoneal and Intravesical Bacillus Calmette-Guerin on Bladder Carcinogenesis in Rats Induced by N-butyl-N-(4-hydroxybutyl) nitrosamine