J Clin Neurol.
2012 Dec;8(4):284-292. 10.3988/jcn.2012.8.4.284.
Supra-Additive Neuroprotection by Renexin, a Mixed Compound of Ginkgo Biloba Extract and Cilostazol, Against Apoptotic White Matter Changes in Rat after Chronic Cerebral Hypoperfusion
- Affiliations
-
- 1Department of Biomedical Science, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 2Department of Neurology, College of Medicine, The Catholic University of Korea, Seoul, Korea. nuyikim@catholic.ac.kr
- KMID: 2287580
- DOI: http://doi.org/10.3988/jcn.2012.8.4.284
Abstract
- BACKGROUND AND PURPOSE
White-matter (WM) lesions are known to potentiate cognitive impairment in poststroke patients. The present study was designed to assess whether Ginkgo biloba extract (GB) and cilostazol, which were evaluated alone and in a combination formula (Renexin), can attenuate the WM lesions and cognitive decline caused by chronic hypoperfusion in the rat.
METHODS
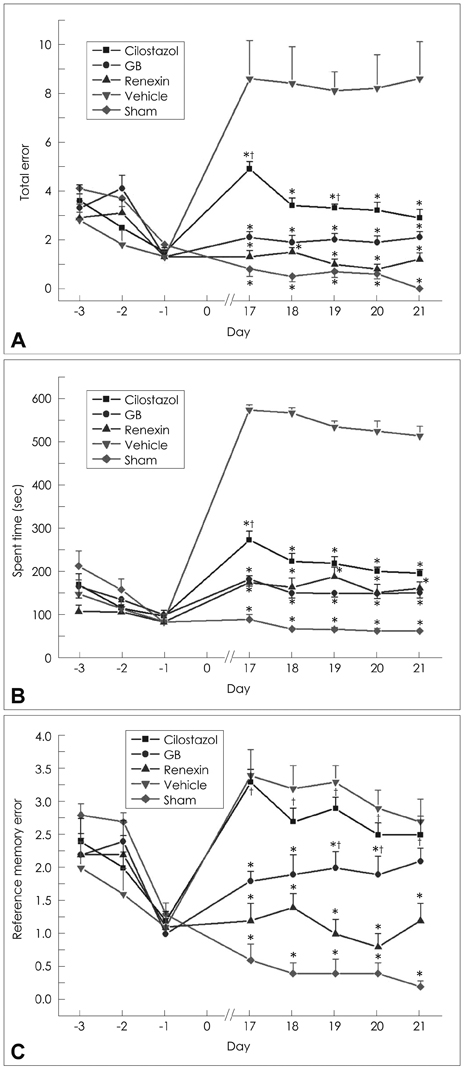
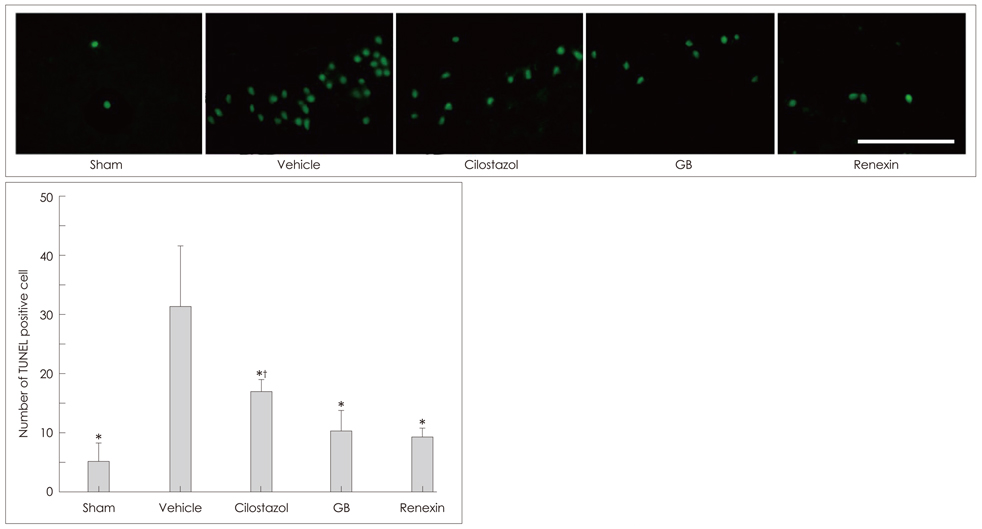
Animals were divided into five treatment groups: cilostazol (25 mg/kg/day), GB (20 mg/kg/day), Renexin (25 mg/kg/day cilostazol + 20 mg/kg/day GB), vehicle, and sham. The animals received the treatments orally 1 day after bilateral common carotid artery occlusion [two-vessel occlusion (2VO); except for the sham group, which underwent the surgery but the arteries were not occluded], and then the same dose every day for 21 days thereafter. Prior to sacrificing the rats, repetitive eight-arm radial maze testing was performed to examine their cognitive abilities. After drug administration and cognitive testing, brain tissues were isolated for Kluver-Barrera and terminal deoxynucleotidyl transferase-mediated biotin-dUTP nick end-labeling (TUNEL) staining, immunohistochemical assessment of glial fibrillary acidic protein (GFAP) and CD11b (OX-42), and to assay free-radical scavenging activity.
RESULTS
We found that the significant WM lesions induced by 2VO was ameliorated significantly by treatment with cilostazol, GB, and Renexin, in association with increased TUNEL-positive cells. In addition, chronic cerebral hypoperfusion caused a large increase in the degree of GFAP and OX-42 immunoreactivity and free-radical activity in the optic tract. These abnormalities were significantly reversed by the three drugs, but most prominently by Renexin, suggesting a markedly enhanced or supra-additive effect of cilostazol and GB when administered together.
CONCLUSIONS
Significant attenuation of cytoarchitectural damage and apoptotic cell death was found with GB and cilostazol, but a markedly enhanced effect was seen for treatment with their combination in the WM of rat brains after bilateral occlusion of the common carotid arteries. We suggest that combination therapy with GB and cilostazol provides enhanced neuroprotective effects and induces subsequent cognitive improvement in patients with chronic ischemic conditions.
MeSH Terms
Figure
Reference
-
1. Beal MF, Hyman BT, Koroshetz W. Do defects in mitochondrial energy metabolism underlie the pathology of neurodegenerative diseases? Trends Neurosci. 1993. 16:125–131.
Article2. Kim JS, Yun I, Choi YB, Lee KS, Kim YI. Ramipril protects from free radical induced white matter damage in chronic hypoperfusion in the rat. J Clin Neurosci. 2008. 15:174–178.
Article3. O'Brien MD. How does cerebrovascular disease cause dementia? Dementia. 1994. 5:133–136.4. Bennett SA, Tenniswood M, Chen JH, Davidson CM, Keyes MT, Fortin T, et al. Chronic cerebral hypoperfusion elicits neuronal apoptosis and behavioral impairment. Neuroreport. 1998. 9:161–166.
Article5. Nanri M, Miyake H, Murakami Y, Matsumoto K, Watanabe H. Chronic cerebral hypoperfusion-induced neuropathological changes in rats. Nihon Shinkei Seishin Yakurigaku Zasshi. 1998. 18:181–188.6. Szabo ME, Droy-Lefaix MT, Doly M. EGb 761 and the recovery of ion imbalance in ischemic reperfused diabetic rat retina. Ophthalmic Res. 1995. 27:102–109.
Article7. Pincemail J, Dupuis M, Nasr C, Hans P, Haag-Berrurier M, Anton R, et al. Superoxide anion scavenging effect and superoxide dismutase activity of Ginkgo biloba extract. Experientia. 1989. 45:708–712.
Article8. Varga E, Bodi A, Ferdinandy P, Droy-Lefaix MT, Blasig IE, Tosaki A. The protective effect of EGb 761 in isolated ischemic/reperfused rat hearts: a link between cardiac function and nitric oxide production. J Cardiovasc Pharmacol. 1999. 34:711–717.
Article9. Kimura Y, Tani T, Kanbe T, Watanabe K. Effect of cilostazol on platelet aggregation and experimental thrombosis. Arzneimittelforschung. 1985. 35:1144–1149.10. Kim KY, Shin HK, Choi JM, Hong KW. Inhibition of lipopolysaccharide-induced apoptosis by cilostazol in human umbilical vein endothelial cells. J Pharmacol Exp Ther. 2002. 300:709–715.
Article11. Shin HK, Kim YK, Kim KY, Lee JH, Hong KW. Remnant lipoprotein particles induce apoptosis in endothelial cells by NAD(P)H oxidase-mediated production of superoxide and cytokines via lectin-like oxidized low-density lipoprotein receptor-1 activation: prevention by cilostazol. Circulation. 2004. 109:1022–1028.
Article12. Choi JM, Shin HK, Kim KY, Lee JH, Hong KW. Neuroprotective effect of cilostazol against focal cerebral ischemia via antiapoptotic action in rats. J Pharmacol Exp Ther. 2002. 300:787–793.
Article13. Lee JH, Lee YK, Ishikawa M, Koga K, Fukunaga M, Miyakoda G, et al. Cilostazol reduces brain lesion induced by focal cerebral ischemia in rats--an MRI study. Brain Res. 2003. 994:91–98.
Article14. Davidson CM, Pappas BA, Stevens WD, Fortin T, Bennett SA. Chronic cerebral hypoperfusion: loss of pupillary reflex, visual impairment and retinal neurodegeneration. Brain Res. 2000. 859:96–103.
Article15. Paxinos G. The Rat Brain in Stereotaxic Coordinates. 2007. 6th ed. San Diego: Academic Press.16. Wakita H, Tomimoto H, Akiguchi I, Kimura J. Dose-dependent, protective effect of FK506 against white matter changes in the rat brain after chronic cerebral ischemia. Brain Res. 1998. 792:105–113.
Article17. Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991. 11:81–128.
Article18. Ihara M, Tomimoto H, Kinoshita M, Oh J, Noda M, Wakita H, et al. Chronic cerebral hypoperfusion induces MMP-2 but not MMP-9 expression in the microglia and vascular endothelium of white matter. J Cereb Blood Flow Metab. 2001. 21:828–834.
Article19. Murakami Y, Ikenoya M, Matsumoto K, Li H, Watanabe H. Ameliorative effect of tacrine on spatial memory deficit in chronic two-vessel occluded rats is reversible and mediated by muscarinic M1 receptor stimulation. Behav Brain Res. 2000. 109:83–90.
Article20. Tanaka K, Ogawa N, Asanuma M, Kondo Y, Nomura M. Relationship between cholinergic dysfunction and discrimination learning disabilities in Wistar rats following chronic cerebral hypoperfusion. Brain Res. 1996. 729:55–65.
Article21. Wakita H, Tomimoto H, Akiguchi I, Kimura J. Glial activation and white matter changes in the rat brain induced by chronic cerebral hypoperfusion: an immunohistochemical study. Acta Neuropathol. 1994. 87:484–492.
Article22. Tsuchiya M, Sako K, Yura S, Yonemasu Y. Local cerebral glucose utilisation following acute and chronic bilateral carotid artery ligation in Wistar rats: relation to changes in local cerebral blood flow. Exp Brain Res. 1993. 95:1–7.
Article23. van den Heuvel DM, ten Dam VH, de Craen AJ, Admiraal-Behloul F, Olofsen H, Bollen EL, et al. Increase in periventricular white matter hyperintensities parallels decline in mental processing speed in a non-demented elderly population. J Neurol Neurosurg Psychiatry. 2006. 77:149–153.
Article24. Volbracht C, van Beek J, Zhu C, Blomgren K, Leist M. Neuroprotective properties of memantine in different in vitro and in vivo models of excitotoxicity. Eur J Neurosci. 2006. 23:2611–2622.
Article25. Watanabe T, Zhang N, Liu M, Tanaka R, Mizuno Y, Urabe T. Cilostazol protects against brain white matter damage and cognitive impairment in a rat model of chronic cerebral hypoperfusion. Stroke. 2006. 37:1539–1545.
Article26. Eckert A, Keil U, Kressmann S, Schindowski K, Leutner S, Leutz S, et al. Effects of EGb 761 Ginkgo biloba extract on mitochondrial function and oxidative stress. Pharmacopsychiatry. 2003. 36:Suppl 1. S15–S23.
Article27. Ryu KH, Han HY, Lee SY, Jeon SD, Im GJ, Lee BY, et al. Ginkgo biloba extract enhances antiplatelet and antithrombotic effects of cilostazol without prolongation of bleeding time. Thromb Res. 2009. 124:328–334.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Ginkgo Biloba to Retinal Microcirculation
- Protective effects of Ginkgo biloba extract (EGB 761) on astrocytes of rat hippocampus after exposure with scopolamine
- The Effect of Ginkgo Biloba Extract on Diabetic Peripheral Neuropathy - A 12 week, randomized, placebo-controlled, double-blind trial -
- Treatment Effect of Intravenous Lasix-Vitamin-Dextran and Carbogen Inhalation Therapy Accompanying Oral Drug Therapy in the Patients with Tinnitus
- Gingko biloba extract (EGb 761) attenuates ischemic brain injury-induced reduction in Ca2+ sensor protein hippocalcin