Korean J Physiol Pharmacol.
2016 May;20(3):305-314. 10.4196/kjpp.2016.20.3.305.
NecroX-5 exerts anti-inflammatory and anti-fibrotic effects via modulation of the TNFα/Dcn/TGFβ1/Smad2 pathway in hypoxia/reoxygenation-treated rat hearts
- Affiliations
-
- 1National Research Laboratory for Mitochondrial Signaling, Department of Physiology, Department of Health Sciences and Technology, BK21 Project Team, College of Medicine, Cardiovascular and Metabolic Disease Center, Inje University, Busan 47392, Korea. phy
- 2Department of Integrated Biomedical Science, College of Medicine, Inje University, Busan 47392, Korea.
- 3VNU University of Science, Hanoi 120036, Vietnam.
- 4Product Strategy and Development, LG Life Sciences Ltd., Seoul 03184, Korea.
- KMID: 2285603
- DOI: http://doi.org/10.4196/kjpp.2016.20.3.305
Abstract
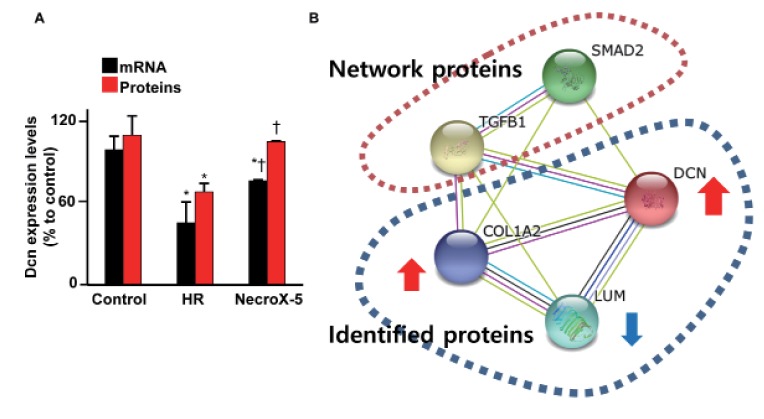
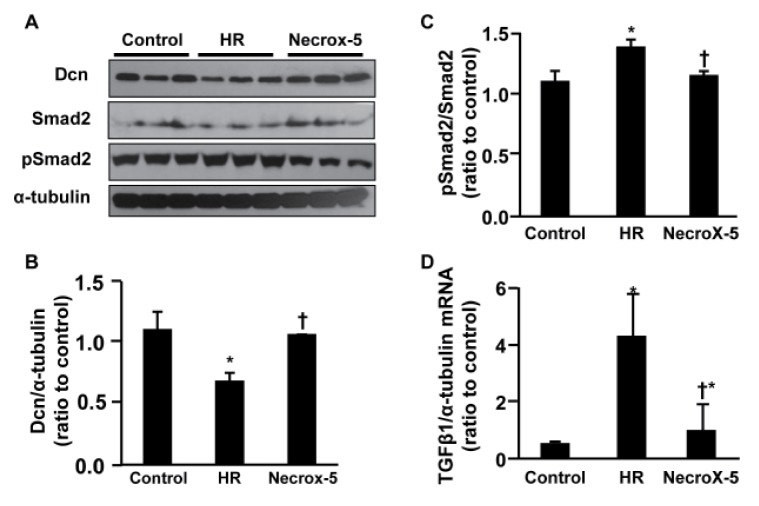
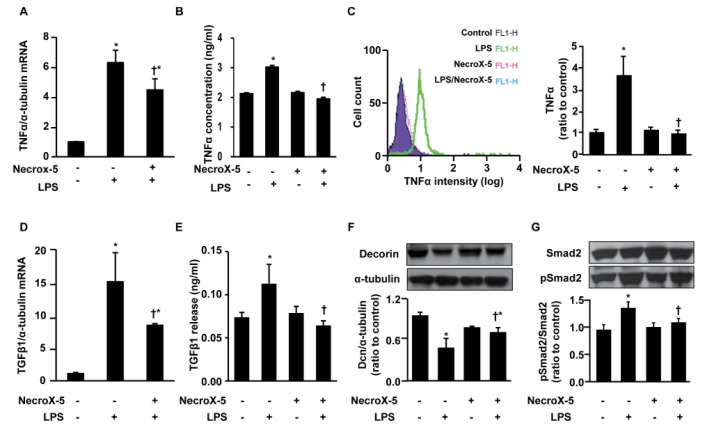
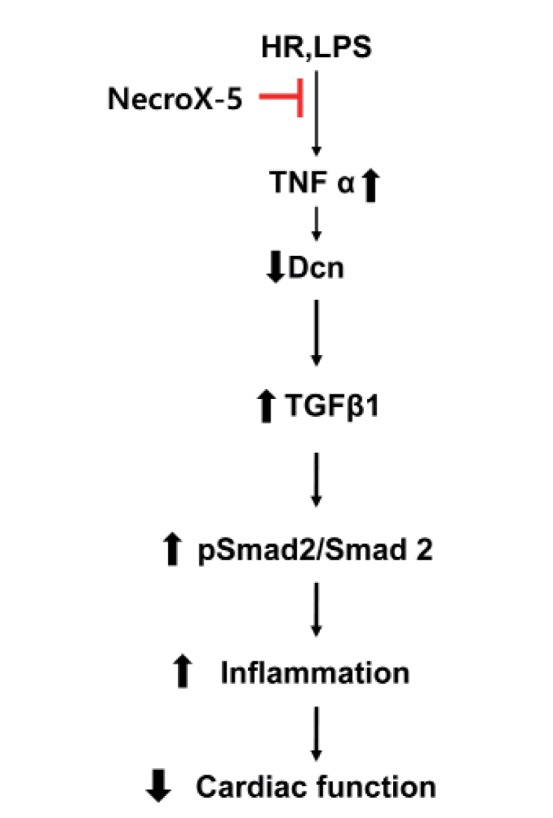
- Inflammatory and fibrotic responses are accelerated during the reperfusion period, and excessive fibrosis and inflammation contribute to cardiac malfunction. NecroX compounds have been shown to protect the liver and heart from ischemia-reperfusion injury. The aim of this study was to further define the role and mechanism of action of NecroX-5 in regulating infl ammation and fi brosis responses in a model of hypoxia/reoxygenation (HR). We utilized HR-treated rat hearts and lipopolysaccharide (LPS)-treated H9C2 culture cells in the presence or absence of NecroX-5 (10 µmol/L) treatment as experimental models. Addition of NecroX-5 signifi cantly increased decorin (Dcn) expression levels in HR-treated hearts. In contrast, expression of transforming growth factor beta 1 (TGFβ1) and Smad2 phosphorylation (pSmad2) was strongly attenuated in NecroX-5-treated hearts. In addition, signifi cantly increased production of tumor necrosis factor alpha (TNFα), TGFβ1, and pSmad2, and markedly decreased Dcn expression levels, were observed in LPS-stimulated H9C2 cells. Interestingly, NecroX-5 supplementation effectively attenuated the increased expression levels of TNFα, TGFβ1, and pSmad2, as well as the decreased expression of Dcn. Thus, our data demonstrate potential antiinflammatory and anti-fibrotic effects of NecroX-5 against cardiac HR injuries via modulation of the TNFα/Dcn/TGFβ1/Smad2 pathway.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Time-dependent proteomic and genomic alterations in Toll-like receptor-4-activated human chondrocytes: increased expression of lamin A/C and annexins
Seung Hee Ha, Hyoung Kyu Kim, Nguyen Thi Tuyet Anh, Nari Kim, Kyung Soo Ko, Byoung Doo Rhee, Jin Han
Korean J Physiol Pharmacol. 2017;21(5):531-546. doi: 10.4196/kjpp.2017.21.5.531.
Reference
-
1. Halestrap AP. A pore way to die: the role of mitochondria in reperfusion injury and cardioprotection. Biochem Soc Trans. 2010; 38:841–860. PMID: 20658967.
Article2. Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol. 2000; 190:255–266. PMID: 10685060.
Article3. Kleinbongard P, Schulz R, Heusch G. TNFα in myocardial ischemia/reperfusion, remodeling and heart failure. Heart Fail Rev. 2011; 16:49–69. PMID: 20571888.
Article4. Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004; 94:1543–1553. PMID: 15217919.
Article5. Schulz R, Aker S, Belosjorow S, Heusch G. TNFalpha in ischemia/ reperfusion injury and heart failure. Basic Res Cardiol. 2004; 99:8–11. PMID: 14685700.6. Bujak M, Frangogiannis NG. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res. 2007; 74:184–195. PMID: 17109837.7. Mosca S, Gargiulo P, Balato N, Di Costanzo L, Parente A, Paolillo S, Ayala F, Trimarco B, Crea F, Perrone-Filardi P. Ischemic cardiovascular involvement in psoriasis: a systematic review. Int J Cardiol. 2015; 178:191–199. PMID: 25464252.
Article8. Kuwahara F, Kai H, Tokuda K, Kai M, Takeshita A, Egashira K, Imaizumi T. Transforming growth factor-beta function blocking prevents myocardial fibrosis and diastolic dysfunction in pressureoverloaded rats. Circulation. 2002; 106:130–135. PMID: 12093782.9. Lijnen PJ, Petrov VV, Fagard RH. Induction of cardiac fibrosis by transforming growth factor-beta(1). Mol Genet Metab. 2000; 71:418–435. PMID: 11001836.10. Mauviel A, Santra M, Chen YQ, Uitto J, Iozzo RV. Transcriptional regulation of decorin gene expression. Induction by quiescence and repression by tumor necrosis factor-alpha. J Biol Chem. 1995; 270:11692–11700. PMID: 7744809.11. Beanes SR, Dang C, Soo C, Wang Y, Urata M, Ting K, Fonkalsrud EW, Benhaim P, Hedrick MH, Atkinson JB, Lorenz HP. Down-regulation of decorin, a transforming growth factor-beta modulator, is associated with scarless fetal wound healing. J Pediatr Surg. 2001; 36:1666–1671. PMID: 11685698.
Article12. Edwards IJ, Xu H, Wright MJ, Wagner WD. Interleukin-1 up-regulates decorin production by arterial smooth muscle cells. Arterioscler Thromb. 1994; 14:1032–1039. PMID: 8018657.
Article13. Mauviel A, Korang K, Santra M, Tewari D, Uitto J, Iozzo RV. Identification of a bimodal regulatory element encompassing a canonical AP-1 binding site in the proximal promoter region of the human decorin gene. J Biol Chem. 1996; 271:24824–24829. PMID: 8798756.
Article14. Järvinen TA, Ruoslahti E. Target-seeking antifibrotic compound enhances wound healing and suppresses scar formation in mice. Proc Natl Acad Sci U S A. 2010; 107:21671–21676. PMID: 21106754.15. Steffens S, Montecucco F, Mach F. The inflammatory response as a target to reduce myocardial ischaemia and reperfusion injury. Thromb Haemost. 2009; 102:240–247. PMID: 19652874.
Article16. Mohan RR, Tandon A, Sharma A, Cowden JW, Tovey JC. Significant inhibition of corneal scarring in vivo with tissue-selective, targeted AAV5 decorin gene therapy. Invest Ophthalmol Vis Sci. 2011; 52:4833–4841. PMID: 21551414.
Article17. Jahanyar J, Joyce DL, Southard RE, Loebe M, Noon GP, Koerner MM, Torre-Amione G, Youker KA. Decorin-mediated transforming growth factor-beta inhibition ameliorates adverse cardiac remodeling. J Heart Lung Transplant. 2007; 26:34–40. PMID: 17234515.18. Simkhovich BZ, Marjoram P, Poizat C, Kedes L, Kloner RA. Age-related changes of cardiac gene expression following myocardial ischemia/reperfusion. Arch Biochem Biophys. 2003; 420:268–278. PMID: 14654066.
Article19. Doi M, Kusachi S, Murakami T, Ninomiya Y, Murakami M, Nakahama M, Takeda K, Komatsubara I, Naito I, Tsuji T. Time-dependent changes of decorin in the infarct zone after experimentally induced myocardial infarction in rats: comparison with biglycan. Pathol Res Pract. 2000; 196:23–33. PMID: 10674269.
Article20. Hao J, Ju H, Zhao S, Junaid A, Scammell-La Fleur T, Dixon IM. Elevation of expression of Smads 2, 3, and 4, decorin and TGF-beta in the chronic phase of myocardial infarct scar healing. J Mol Cell Cardiol. 1999; 31:667–678. PMID: 10198196.21. Weis SM, Zimmerman SD, Shah M, Covell JW, Omens JH, Ross J Jr, Dalton N, Jones Y, Reed CC, Iozzo RV, McCulloch AD. A role for decorin in the remodeling of myocardial infarction. Matrix Biol. 2005; 24:313–324. PMID: 15949932.
Article22. Li L, Okada H, Takemura G, Kosai K, Kanamori H, Esaki M, Takahashi T, Goto K, Tsujimoto A, Maruyama R, Kawamura I, Kawaguchi T, Takeyama T, Fujiwara T, Fujiwara H, Minatoguchi S. Postinfarction gene therapy with adenoviral vector expressing decorin mitigates cardiac remodeling and dysfunction. Am J Physiol Heart Circ Physiol. 2009; 297:H1504–H1513. PMID: 19684189.
Article23. Burlew BS, Weber KT. Connective tissue and the heart. Functional significance and regulatory mechanisms. Cardiol Clin. 2000; 18:435–442. PMID: 10986582.24. Jalil JE, Doering CW, Janicki JS, Pick R, Shroff SG, Weber KT. Fibrillar collagen and myocardial stiffness in the intact hypertrophied rat left ventricle. Circ Res. 1989; 64:1041–1050. PMID: 2524288.
Article25. Kim HJ, Koo SY, Ahn BH, Park O, Park DH, Seo DO, Won JH, Yim HJ, Kwak HS, Park HS, Chung CW, Oh YL, Kim SH. NecroX as a novel class of mitochondrial reactive oxygen species and ONOO-scavenger. Arch Pharm Res. 2010; 33:1813–1823. PMID: 21116785.26. Piper HM, Garcia-Dorado D. Reducing the impact of myocardial ischaemia/reperfusion injury. Cardiovasc Res. 2012; 94:165–167. PMID: 22461524.
Article27. Choi JM, Park KM, Kim SH, Hwang DW, Chon SH, Lee JH, Lee SY, Lee YJ. Effect of necrosis modulator necrox-7 on hepatic ischemiareperfusion injury in beagle dogs. Transplant Proc. 2010; 42:3414–3421. PMID: 21094788.
Article28. Song JJ, Chang J, Choi J, Im GJ, Chae SW, Lee SH, Kwon SY, Jung HH, Chung AY, Park HC, Choi J. Protective role of NecroX-5 against neomycin-induced hair cell damage in zebrafish. Arch Toxicol. 2014; 88:435–441. PMID: 24030356.
Article29. Lee SR, Lee SJ, Kim SH, Ko KS, Rhee BD, Xu Z, Kim N, Han J. NecroX-5 suppresses sodium nitroprusside-induced cardiac cell death through inhibition of JNK and caspase-3 activation. Cell Biol Int. 2014; 38:702–707. PMID: 24446382.
Article30. Park MK, Lee BD, Chae SW, Chi J, Kwon SK, Song JJ. Protective effect of NecroX, a novel necroptosis inhibitor, on gentamicininduced ototoxicity. Int J Pediatr Otorhinolaryngol. 2012; 76:1265–1269. PMID: 22704672.
Article31. Thu VT, Kim HK, Long le T, Lee SR, Hanh TM, Ko TH, Heo HJ, Kim N, Kim SH, Ko KS, Rhee BD, Han J. NecroX-5 prevents hypoxia/reoxygenation injury by inhibiting the mitochondrial calcium uniporter. Cardiovasc Res. 2012; 94:342–350. PMID: 22425903.
Article32. Takaseya T, Ishimatsu M, Tayama E, Nishi A, Akasu T, Aoyagi S. Mechanical unloading improves intracellular Ca2+ regulation in rats with doxorubicin-induced cardiomyopathy. J Am Coll Cardiol. 2004; 44:2239–2246. PMID: 15582323.33. Kim N, Lee Y, Kim H, Joo H, Youm JB, Park WS, Warda M, Cuong DV, Han J. Potential biomarkers for ischemic heart damage identified in mitochondrial proteins by comparative proteomics. Proteomics. 2006; 6:1237–1249. PMID: 16402359.
Article34. Ding G, Cheng L, Qin Q, Frontin S, Yang Q. PPARdelta modulates lipopolysaccharide-induced TNFalpha inflammation signaling in cultured cardiomyocytes. J Mol Cell Cardiol. 2006; 40:821–828. PMID: 16698033.35. Thu VT, Kim HK, Long le T, Nyamaa B, Song IS, Thuy TT, Huy NQ, Marquez J, Kim SH, Kim N, Ko KS, Rhee BD2, Han J. NecroX-5 protects mitochondrial oxidative phosphorylation capacity and preserves PGC1α expression levels during hypoxia/reoxygenation injury. Korean J Physiol Pharmacol. 2016; 20:201–211. PMID: 26937217.
Article36. Kim HK, Kim YK, Song IS, Lee SR, Jeong SH, Kim MH, Seo DY, Kim N, Rhee BD, Ko KS, Tark KC, Park CG, Cho JY, Han J. Human giant congenital melanocytic nevus exhibits potential proteomic alterations leading to melanotumorigenesis. Proteome Sci. 2012; 10:50. PMID: 22906024.
Article37. Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, Kuhn M, Bork P, Jensen LJ, von Mering C. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015; 43:D447–D452. PMID: 25352553.
Article38. Bruckner BA, Razeghi P, Stetson S, Thompson L, Lafuente J, Entman M, Loebe M, Noon G, Taegtmeyer H, Frazier OH, Youker K. Degree of cardiac fibrosis and hypertrophy at time of implantation predicts myocardial improvement during left ventricular assist device support. J Heart Lung Transplant. 2004; 23:36–42. PMID: 14734125.
Article39. Bruckner BA, Stetson SJ, Perez-Verdia A, Youker KA, Radovancevic B, Connelly JH, Koerner MM, Entman ME, Frazier OH, Noon GP, Torre-Amione G. Regression of fibrosis and hypertrophy in failing myocardium following mechanical circulatory support. J Heart Lung Transplant. 2001; 20:457–464. PMID: 11295584.
Article40. Zimmerman SD, Thomas DP, Velleman SG, Li X, Hansen TR, McCormick RJ. Time course of collagen and decorin changes in rat cardiac and skeletal muscle post-MI. Am J Physiol Heart Circ Physiol. 2001; 281:H1816–H1822. PMID: 11557576.
Article41. Deten A, Hölzl A, Leicht M, Barth W, Zimmer HG. Changes in extracellular matrix and in transforming growth factor beta isoforms after coronary artery ligation in rats. J Mol Cell Cardiol. 2001; 33:1191–1207. PMID: 11444923.
Article42. Yilmaz S, Canpolat U, Aydogdu S, Abboud HE. Diabetic Cardiomyopathy; Summary of 41 Years. Korean Circ J. 2015; 45:266–272. PMID: 26240579.
Article43. Ma M, Watanabe K, Wahed MI, Inoue M, Sekiguchi T, Kouda T, Ohta Y, Nakazawa M, Yoshida Y, Yamamoto T, Hanawa H, Kodama M, Fuse K, Aizawa Y. Inhibition of progression of heart failure and expression of TGF-beta 1 mRNA in rats with heart failure by the ACE inhibitor quinapril. J Cardiovasc Pharmacol. 2001; 38(Suppl 1):S51–S54. PMID: 11811359.44. Giri SN, Hyde DM, Braun RK, Gaarde W, Harper JR, Pierschbacher MD. Antifibrotic effect of decorin in a bleomycin hamster model of lung fibrosis. Biochem Pharmacol. 1997; 54:1205–1216. PMID: 9416971.
Article45. Costacurta A, Priante G, D'Angelo A, Chieco-Bianchi L, Cantaro S. Decorin transfection in human mesangial cells downregulates genes playing a role in the progression of fibrosis. J Clin Lab Anal. 2002; 16:178–186. PMID: 12112390.
Article46. Isaka Y, Brees DK, Ikegaya K, Kaneda Y, Imai E, Noble NA, Border WA. Gene therapy by skeletal muscle expression of decorin prevents fibrotic disease in rat kidney. Nat Med. 1996; 2:418–423. PMID: 8597951.
Article47. Alan C, Kocoglu H, Altintas R, Alici B, Resit Ersay A. Protective effect of decorin on acute ischaemia-reperfusion injury in the rat kidney. Arch Med Sci. 2011; 7:211–216. PMID: 22291758.
Article48. Yan W, Wang P, Zhao CX, Tang J, Xiao X, Wang DW. Decorin gene delivery inhibits cardiac fibrosis in spontaneously hypertensive rats by modulation of transforming growth factor-beta/Smad and p38 mitogen-activated protein kinase signaling pathways. Hum Gene Ther. 2009; 20:1190–1200. PMID: 19697998.49. Kanzleiter T, Rath M, Görgens SW, Jensen J, Tangen DS, Kolnes AJ, Kolnes KJ, Lee S, Eckel J, Schürmann A, Eckardt K. The myokine decorin is regulated by contraction and involved in muscle hypertrophy. Biochem Biophys Res Commun. 2014; 450:1089–1094. PMID: 24996176.
Article50. Dizon LA, Seo DY, Kim HK, Kim N, Ko KS, Rhee BD, Han J. Exercise perspective on common cardiac medications. Integr Med Res. 2013; 2:49–55. PMID: 28664054.
Article51. Mogyorosi A, Ziyadeh FN. Increased decorin mRNA in diabetic mouse kidney and in mesangial and tubular cells cultured in high glucose. Am J Physiol. 1998; 275:F827–F832. PMID: 9815141.52. Kähäri VM, Häkkinen L, Westermarck J, Larjava H. Differential regulation of decorin and biglycan gene expression by dexamethasone and retinoic acid in cultured human skin fibroblasts. J Invest Dermatol. 1995; 104:503–508. PMID: 7706767.
Article53. Bjørnstad JL, Skrbic B, Marstein HS, Hasic A, Sjaastad I, Louch WE, Florholmen G, Christensen G, Tnnessen T. Inhibition of SMAD2 phosphorylation preserves cardiac function during pressure overload. Cardiovasc Res. 2012; 93:100–110. PMID: 22049534.
Article54. Abdel-Wahab N, Wicks SJ, Mason RM, Chantry A. Decorin suppresses transforming growth factor-beta-induced expression of plasminogen activator inhibitor-1 in human mesangial cells through a mechanism that involves Ca2+-dependent phosphorylation of Smad2 at serine-240. Biochem J. 2002; 362:643–649. PMID: 11879191.55. Kim SR, Kim DI, Kim SH, Lee H, Lee KS, Cho SH, Lee YC. NLRP3 inflammasome activation by mitochondrial ROS in bronchial epithelial cells is required for allergic inflammation. Cell Death Dis. 2014; 5:e1498. PMID: 25356867.
Article56. Cabello-Verrugio C, Brandan E. A novel modulatory mechanism of transforming growth factor-beta signaling through decorin and LRP-1. J Biol Chem. 2007; 282:18842–18850. PMID: 17485468.57. Naik E, Dixit VM. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J Exp Med. 2011; 208:417–420. PMID: 21357740.
Article58. Hunter RL, Dragicevic N, Seifert K, Choi DY, Liu M, Kim HC, Cass WA, Sullivan PG, Bing G. Inflammation induces mitochondrial dysfunction and dopaminergic neurodegeneration in the nigrostriatal system. J Neurochem. 2007; 100:1375–1386. PMID: 17254027.
Article59. López-Armada MJ, Riveiro-Naveira RR, Vaamonde-García C, Valcárcel-Ares MN. Mitochondrial dysfunction and the inflammatory response. Mitochondrion. 2013; 13:106–118. PMID: 23333405.
Article60. Dada LA, Sznajder JI. Mitochondrial Ca2+ and ROS take center stage to orchestrate TNF-α-mediated inflammatory responses. J Clin Invest. 2011; 121:1683–1685. PMID: 21519140.61. McLeod CJ, Pagel I, Sack MN. The mitochondrial biogenesis regulatory program in cardiac adaptation to ischemia--a putative target for therapeutic intervention. Trends Cardiovasc Med. 2005; 15:118–123. PMID: 16039972.
Article62. Groeneveld TW, Oroszlán M, Owens RT, Faber-Krol MC, Bakker AC, Arlaud GJ, McQuillan DJ, Kishore U, Daha MR, Roos A. Interactions of the extracellular matrix proteoglycans decorin and biglycan with C1q and collectins. J Immunol. 2005; 175:4715–4723. PMID: 16177119.
Article63. Marquez J, Lee SR, Kim N, Han J. Post-translational modifications of cardiac mitochondrial proteins in cardiovascular disease: not lost in translation. Korean Circ J. 2016; 46:1–12. PMID: 26798379.
Article64. Ständer M, Naumann U, Wick W, Weller M. Transforming growth factor-beta and p-21: multiple molecular targets of decorin-mediated suppression of neoplastic growth. Cell Tissue Res. 1999; 296:221–227. PMID: 10382266.65. Chandel NS, Trzyna WC, McClintock DS, Schumacker PT. Role of oxidants in NF-kappa B activation and TNF-alpha gene transcription induced by hypoxia and endotoxin. J Immunol. 2000; 165:1013–1021. PMID: 10878378.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- NecroX-5 protects mitochondrial oxidative phosphorylation capacity and preserves PGC1alpha expression levels during hypoxia/reoxygenation injury
- Effects of Hypoxia/Reoxygenation and Sodium Nitroprusside on Acid Secretion and Cyclic Nucleotide of Isolated Gastric Cells
- The Effect of Repetitive Hypoxia on Production of Lipid Peroxidation in Newborn Rat Brain
- Cardioprotection Via Modulation of Calcium Homeostasis by Thiopental in Hypoxia-Reoxygenated Neonatal Rat Cardiomyocytes
- The free radical scavenger NecroX-7 ameliorates tacrolimusinduced pancreatic β cell dysfunction