Yonsei Med J.
2010 Mar;51(2):187-196. 10.3349/ymj.2010.51.2.187.
Cardioprotection Via Modulation of Calcium Homeostasis by Thiopental in Hypoxia-Reoxygenated Neonatal Rat Cardiomyocytes
- Affiliations
-
- 1Department of Life Science, College of Natural Sciences, Ewha Womans University, Seoul, Korea.
- 2Cardiovascular Research Institute, Cardiology Division, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Anesthesiology and Pain Medicine, Anesthesia and Pain Research Institute, Yonsei University College of Medicine, Seoul, Korea. wkp7ark@yuhs.ac
- KMID: 1126016
- DOI: http://doi.org/10.3349/ymj.2010.51.2.187
Abstract
- PURPOSE
Ca2+ homeostasis plays an important role in myocardial cell injury induced by hypoxia-reoxygenation, and prevention of intracellular Ca2+ overload is key to cardioprotection. Even though thiopental is a frequently used anesthetic agent, little is known about its cardioprotective effects, particulary in association with Ca2+ homeostasis. We investigated whether thiopental protects cardiomyocytes against hypoxia-reoxygenation injury by regulating Ca2+ homeostasis.
MATERIALS AND METHODS
Neonatal rat cardiomyocytes were isolated. Cardiomyocytes were exposed to different concentrations of thiopental and immediately replaced in the hypoxic chamber to maintain hypoxia. After 1 hour of exposure, a culture dish was transferred to the CO2 incubator and cells were incubated at 37degrees C for 5 hours. At the end of the experiments, the authors assessed cell protection using immunoblot analysis and caspase activity. The mRNA of genes involved in Ca2+ homeostasis, mitochondrial membrane potential, and cellular Ca2+ levels were examined.
RESULTS
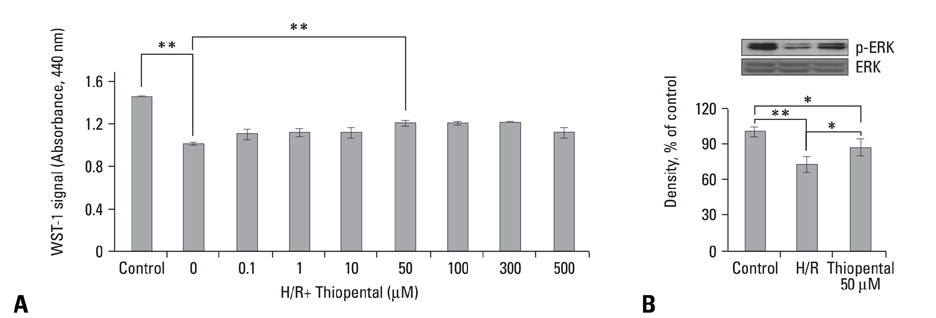
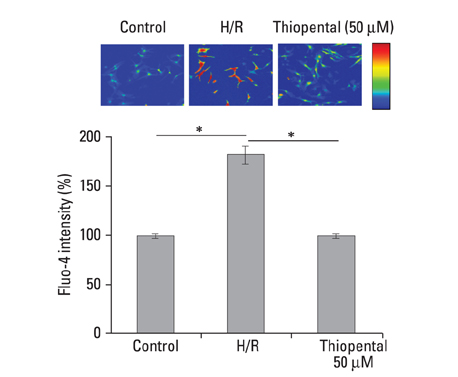
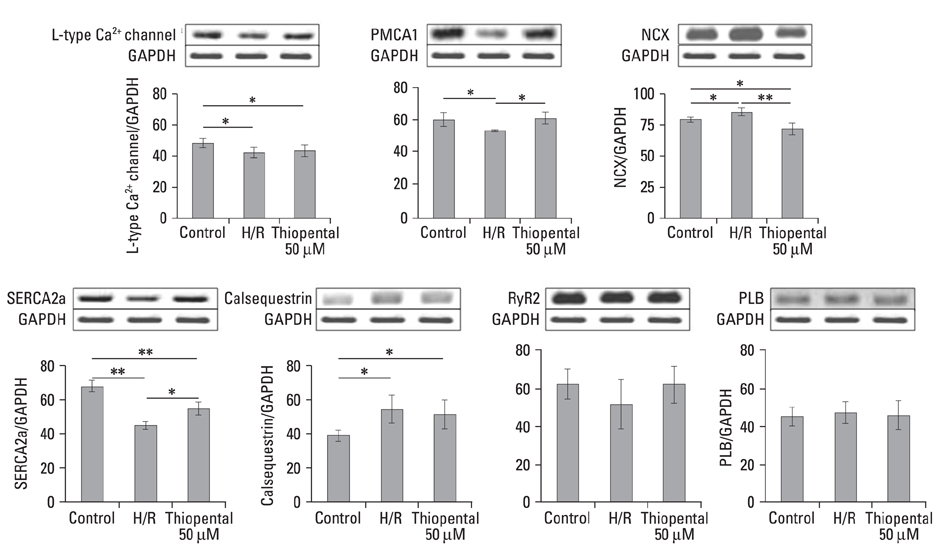
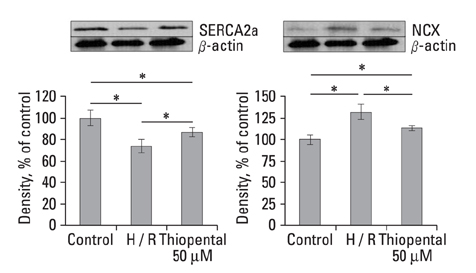
In thiopental-treated cardiomyocytes, there was a decrease in expression of the proapoptotic protein Bax, caspase-3 activation, and intracellular Ca2+ content. In addition, both enhancement of anti-apoptotic protein Bcl-2 and activation of Erk concerned with survival were shown. Furthermore, thiopental attenuated alterations of genes involving Ca2+ regulation and significantly modulated abnormal changes of NCX and SERCA2a genes in hypoxia-reoxygenated neonatal cardiomyocytes. Thiopental suppressed disruption of mitochondrial membrane potential (Delta Psi m) induced by hypoxia-reoxygenation.
CONCLUSION
Thiopental is likely to modulate expression of genes that regulate Ca2+ homeostasis, which reduces apoptotic cell death and results in cardioprotection.
MeSH Terms
-
Animals
Apoptosis
Calcium/*metabolism
Cell Hypoxia/*physiology
Cell Survival/drug effects
Cells, Cultured
GABA Modulators/*pharmacology
Homeostasis/drug effects
Immunoblotting
In Situ Nick-End Labeling
Membrane Potential, Mitochondrial/drug effects
Microscopy, Confocal
Myocytes, Cardiac/*drug effects/*metabolism
Rats
Rats, Sprague-Dawley
Reverse Transcriptase Polymerase Chain Reaction
Thiopental/*pharmacology
Figure
Reference
-
1. Fliss H, Gattinger D. Apoptosis in ischemic and reperfused rat myocardium. Circ Res. 1996. 79:949–956.
Article2. Anaya-Prado R, Toledo-Pereyra LH. The molecular events underlying ischemia/reperfusion injury. Transplant Proc. 2002. 34:2518–2519.
Article3. Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986. 74:1124–1136.4. Marber MS, Latchman DS, Walker JM, Yellon DM. Cardiac stress protein elevation 24 hours after brief ischemia or heat stress is associated with resistance to myocardial infarction. Circulation. 1993. 88:1264–1272.
Article5. Zaugg M, Schaub MC. Signaling and cellular mechanisms in cardiac protection by ischemic and pharmacological preconditioning. J Muscle Res Cell Motil. 2003. 24:219–249.6. Javadov SA, Lim KH, Kerr PM, Suleiman MS, Angelini GD, Halestrap AP. Protection of hearts from reperfusion injury by propofol is associated with inhibition of the mitochondrial permeability transition. Cardiovasc Res. 2000. 45:360–369.7. Schultz JE, Gross GJ. Opioids and cardioprotection. Pharmacol Ther. 2001. 89:123–137.8. Tanaka K, Ludwig LM, Kersten JR, Pagel PS, Warltier DC. Mechanisms of cardioprotection by volatile anesthetics. Anesthesiology. 2004. 100:707–721.
Article9. Kato R, Foëx P. Myocardial protection by anesthetic agents against ischemia-reperfusion injury: an update for anesthesiologists. Can J Anaesth. 2002. 49:777–791.10. Zaugg M, Lucchinetti E, Uecker M, Pasch T, Schaub MC. Anaesthetics and cardiac preconditioning. Part I. Signalling and cytoprotective mechanisms. Br J Anaesth. 2003. 91:551–565.11. Tani M. Mechanisms Ca2+ overload in reperfused ischemic myocardium. Annu Rev Physiol. 1990. 52:543–559.12. Nishina K, Akamatsu H, Mikawa K, Shiga M, Maekawa N, Obara H, et al. The inhibitory effects of thiopental, midazolam, and ketamine on human neutrophil functions. Anesth Analg. 1998. 86:159–165.
Article13. Almaas R, Saugstad OD, Pleasure D, Rootwelt T. Effect of barbiturates on hydroxyl radicals, lipid peroxidation, and hypoxic cell death in human NT2-N neurons. Anesthesiology. 2000. 92:764–774.
Article14. Sano T, Patel PM, Drummond JC, Cole DJ. A comparison of the cerebral protective effects of etomidate, thiopental, and isoflurane in a model of forebrain ischemia in the rat. Anesth Analg. 1993. 76:990–997.
Article15. Sasaki R, Hirota K, Roth SH, Yamazaki M. Anoxic depolarization of rat hippocampal slices is prevented by thiopental but not by propofol or isoflurane. Br J Anaesth. 2005. 94:486–491.
Article16. Sinclair DM, de Moes D, Boink AB, Ruigrok TJ. A protective effect of thiopentone on hypoxic heart muscle. J Mol Cell Cardiol. 1980. 12:225–227.
Article17. Komai H, Berkoff HA, Rusy BF. Protection of ischemic rat heart by cardioplegic doses of pentobarbital. J Surg Res. 1981. 30:42–46.
Article18. Sun HY, Wang NP, Kerendi F, Halkos M, Kin H, Guyton RA, et al. Hypoxic postconditioning reduces cardiomyocyte loss by inhibiting ROS generation and intracellular Ca2+ overload. Am J Physiol Heart Circ Physiol. 2005. 288:H1900–H1908.19. Wang HC, Zhang HF, Guo WY, Su H, Zhang KR, Li QX, et al. Hypoxic postconditioning enhances the survival and inhibits apoptosis of cardiomyocytes following reoxygenation: role of peroxynitrite formation. Apoptosis. 2006. 11:1453–1460.20. Kim HS, Chang WC, Hwang KC, Choi IG, Park WK. Effect of propofol on calcium homeostasis in hypoxia-reoxygenated neonatal rat cardiomyocytes. Eur J Pharmacol. 2008. 594:139–145.
Article21. Park WK, Lynch C 3rd. Propofol and thiopental depression of myocardial contractility. A comparative study of mechanical and electrophysiologic effects in isolated guinea pig ventricular muscle. Anesth Analg. 1992. 74:395–405.22. Cossarizza A, Baccarani-Contri M, Kalashnikova G, Franceschi C. A new method for the cytofluorimetric analysis of mitochondrial membrane potential using the J-aggregate forming lipophillic cation 5,5',6,6'-tetrachloro-1,1',3,3'-tetraethylbenzimidazolcarbocyanine iodine (JC-1). Biochem Biophys Res Commun. 1993. 197:40–45.
Article23. Smiley ST, Reers M, Mottola-Hartshorn C, Lin M, Chen A, Smith TW, et al. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc Natl Acad Sci U S A. 1991. 88:3671–3675.
Article24. Yue TL, Wang C, Gu JL, Ma XL, Kumar S, Lee JC, et al. Inhibition of extracellular signal-regulated kinase enhances Ischemia/Reoxygenation-induced apoptosis in cultured cardiac myocytes and exaggerates reperfusion injury in isolated perfused heart. Circ Res. 2000. 86:692–699.
Article25. de Moissac D, Gurevich RM, Zheng H, Singal PK, Kirshenbaum LA. Caspase activation and mitochondrial cytochrome C release during hypoxia-mediated apoptosis of adult ventricular myocytes. J Mol Cell Cardiol. 2000. 32:53–63.26. Shibuta S, Kosaka J, Mashimo T, Fukuda Y, Yoshiya I. Nitric oxide-induced cytotoxicity attenuation by thiopentone sodium but not pentobarbitone sodium in primary brain cultures. Br J Pharmacol. 1998. 124:804–810.27. Shibuta S, Varathan S, Mashimo T. Ketamine and thiopental sodium: individual and combined neuroprotective effects on cortical cultures exposed to NMDA or nitric oxide. Br J Anaesth. 2006. 97:517–524.
Article28. Basagan-Mogol E, Büyükuysal RL, Korfail G. Effects of ketamine and thiopental on ischemia reoxygenation-induced LDH leakage and amino acid release from rat striatal slices. J Neurosurg Anesthesiol. 2005. 17:20–26.29. Müllenheim J, Molojavyi A, Preckel B, Thämer V, Schlack W. Thiopentone does not block ischemic preconditioning in the isolated rat heart. Can J Anaesth. 2001. 48:784–789.
Article30. Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischemia-reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc Res. 2004. 61:448–460.31. Tsuruta F, Masuyama N, Gotoh Y. The phosphatidylinositol 3-kinase (PI3K)-Akt pathway suppresses Bax translocation to mitochondria. J Biol Chem. 2002. 277:14040–14047.
Article32. Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997. 275:1132–1136.
Article33. Zhu L, Yu Y, Chua BH, Ho YS, Kuo TH. Regulation of sodium-calcium exchange and mitochondrial energetics by Bcl-2 in the heart of transgenic mice. J Mol Cell Cardiol. 2001. 33:2135–2144.
Article34. Dong JW, Zhu HF, Zhu WZ, Ding HL, Ma TM, Zhou ZN. Intermittent hypoxia attenuates ischemia/reperfusion induced apoptosis in cardiac myocytes via regulating Bcl-2/Bax expression. Cell Res. 2003. 13:385–391.
Article35. Pan Z, Bhat MB, Nieminen AL, Ma J. Synergistic movements of Ca2+ and Bax in cells undergoing apoptosis. J Biol Chem. 2001. 276:32257–32263.36. Bers DM. Calcium and cardiac rhythms: physiological and pathophysiological. Circ Res. 2002. 90:14–17.37. Sun HY, Wang NP, Halkos ME, Kerendi F, Kin H, Wang RX, et al. Involvement of Na+/H+ exchanger in hypoxia/re-oxygenation-induced neonatal rat cardiomyocyte apoptosis. Eur J Pharmacol. 2004. 486:121–131.
Article38. Hasenfuss G. Alterations of calcium-regulatory proteins in heart failure. Cardiovasc Res. 1998. 37:279–289.
Article39. Lehotský J, Kaplán P, Racay P, Mézesova V, Raeymaekers L. Distribution of plasma membrane Ca2+ pump (PMCA) isoforms in the gerbil brain: effect of ischemia-reperfusion injury. Neurochem Int. 1999. 35:221–227.
Article40. Imahashi K, Pott C, Goldhaber JI, Steenbergen C, Philipson KD, Murphy E. Cardiac-specific ablation of the Na+-Ca2+ exchanger confers protection against ischemia/reperfusion injury. Circ Res. 2005. 97:916–921.
Article41. Nicoll DA, Longoni S, Philipson KD. Molecular cloning and functional expression of the cardiac sarcolemmal Na+-Ca2+ exchanger. Science. 1990. 250:562–565.
Article42. Miller SL, Currie S, Loughrey CM, Kettlewell S, Seidler T, Reynolds DF, et al. Effects of calsequestrin over-expression on excitation-contraction coupling in isolated rabbit cardiomyocytes. Cardiovasc Res. 2005. 67:667–677.
Article43. Temsah RM, Netticadan T, Chapman D, Takeda S, Mochizuki S, Dhalla NS. Alterations in sarcoplasmic reticulum function and gene expression in ischemic-reperfused rat heart. Am J Physiol. 1999. 277:H584–H594.44. Temsah RM, Dyck C, Netticadan T, Chapman D, Elimban V, Dhalla NS. Effect of beta-adrenoceptor blockers on sarcoplasmic reticular function and gene expression in the ischemic-reperfused heart. J Pharmacol Exp Ther. 2000. 293:15–23.45. Knöll R, Arras M, Zimmermann R, Schaper J, Schaper W. Changes in gene expression following short coronary occlusions studied in porcine hearts with run-on assays. Cardiovasc Res. 1994. 28:1062–1069.
Article46. O'Rourke B. Mitochondrial ion channels. Annu Rev Physiol. 2006. 69:19–49.47. Hüser J, Blatter LA. Fluctuations in mitochondrial membrane potential caused by repetitive gating of the permeability transition pore. Biochem J. 1999. 343:311–317.
Article48. Di Lisa F, Blank PS, Colonna R, Gambassi G, Silverman HS, Stern MD, et al. Mitochondrial membrane potential in single living adult rat cardiac myocytes exposed to anoxia or metabolic inhibition. J Physiol. 1995. 486:1–13.
Article49. Rakhit RD, Mojet MH, Marber MS, Duchen MR. Mitochondria as targets for nitric oxide-induced protection during simulated ischemia and reoxygenation in isolated neonatal cardiomyocytes. Circulation. 2001. 103:2617–2623.
Article50. Zhu LP, Yu XD, Ling S, Brown RA, Kuo TH. Mitochondrial Ca2+ homeostasis in the regulation of apoptotic and necrotic cell deaths. Cell Calcium. 2000. 28:107–117.
Article51. Zaugg M, Lucchinetti E, Spahn DR, Pasch T, Garcia C, Schaub MC. Differential effects of anesthetics on mitochondrial KATP channel activity and cardiomyocyte protection. Anesthesiology. 2002. 97:15–23.
Article52. Liu TJ, Yeh YC, Ting CT, Lee WL, Wang LC, Lee HW, et al. Ginkgo biloba extract 761 reduces doxorubicin-induced apoptotic damage in rat hearts and neonatal cardiomyocytes. Cardiovasc Res. 2008. 80:227–235.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dendropanax morbifera Extract Protects Cardiomyocytes against Hypoxia/Reoxygenation Injury by Inhibition of Reactive Oxygen Species Generation and Calcium Perturbation
- Neonatal Tetany Caused by Hyperparathyroidism Undetected During Pregnancy
- Nanocurcumin–pyrroloquinoline formulation prevents hypertrophy–induced pathological damage by relieving mitochondrial stress in cardiomyocytes under hypoxic conditions
- A Clinical Report for a Hypoxic Cerebral Hypoxia managed by Combination therapy with Thiopental Sodium and Low-grade Hypothermia
- Mitochondrial Dynamics in the Heart as a Novel Therapeutic Target for Cardioprotection