Korean J Physiol Pharmacol.
2014 Oct;18(5):397-402. 10.4196/kjpp.2014.18.5.397.
Regulatory Effect of 25-hydroxyvitamin D3 on Nitric Oxide Production in Activated Microglia
- Affiliations
-
- 1Korea Food Research Institute, Seongnam 463-746, Korea.
- 2Department of Natural Medicine Resources, Semyung University, Jecheon 390-711, Korea.
- 3Department of Physiology, Biomedical Science Institute and Medical Research Center for Reactive Oxygen Species, School of Medicine, Kyung Hee University, Seoul 130-701, Korea. ywcho@khu.ac.kr
- KMID: 2285538
- DOI: http://doi.org/10.4196/kjpp.2014.18.5.397
Abstract
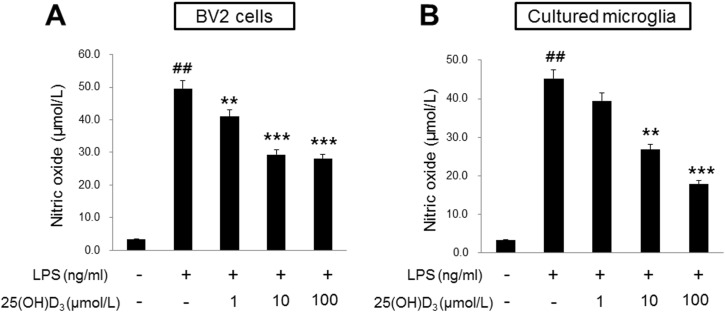
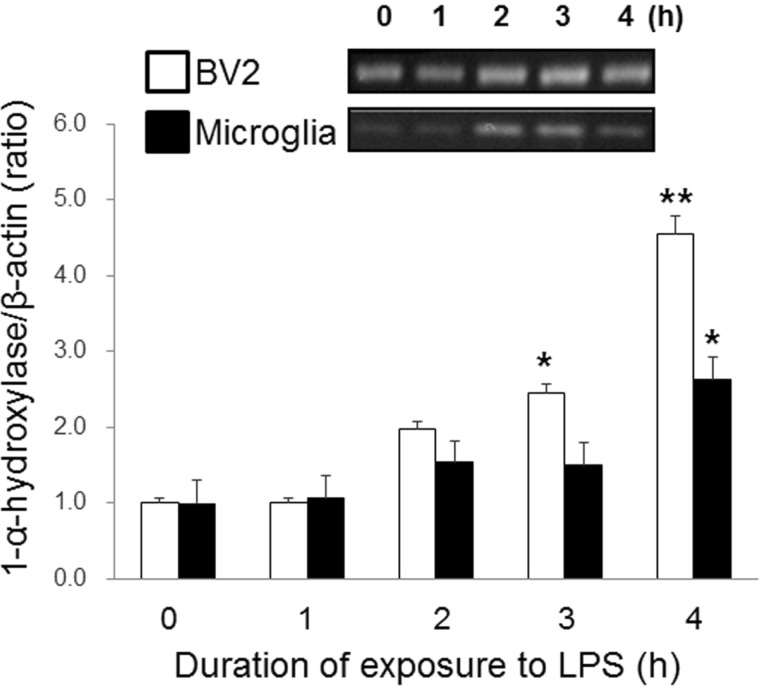
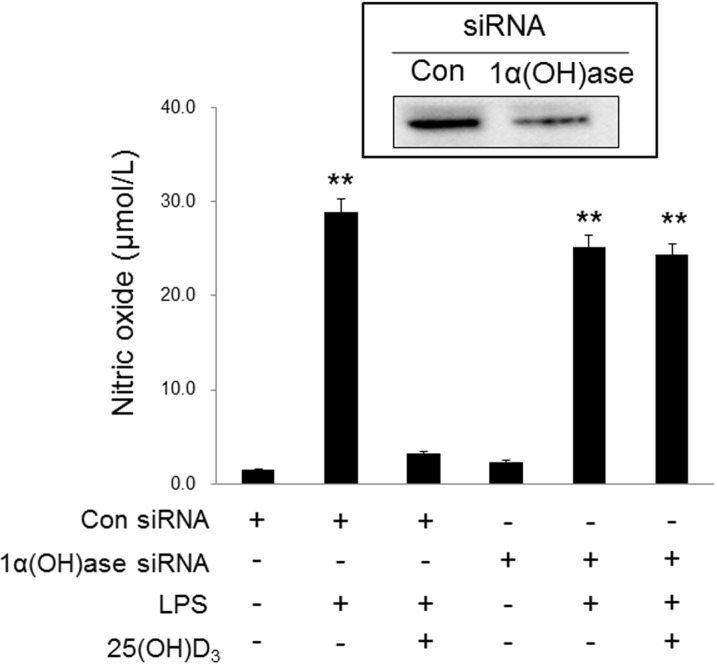
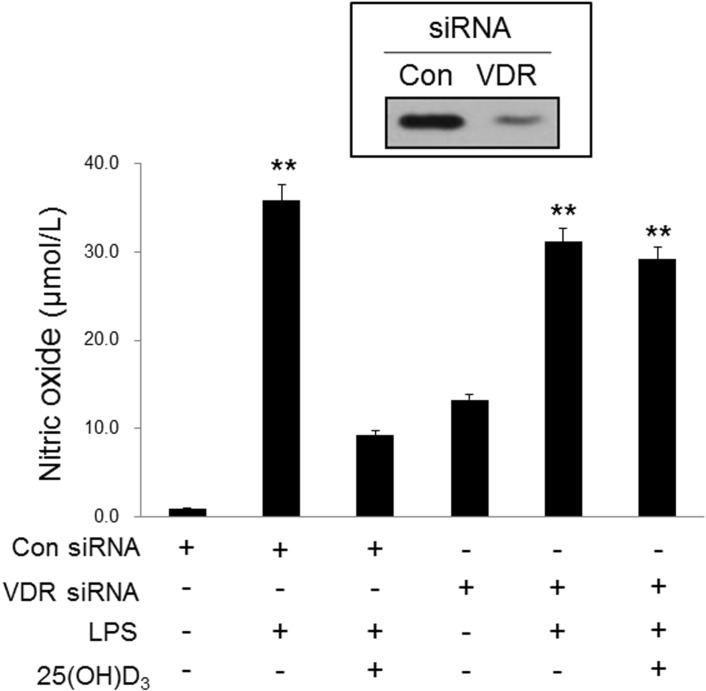
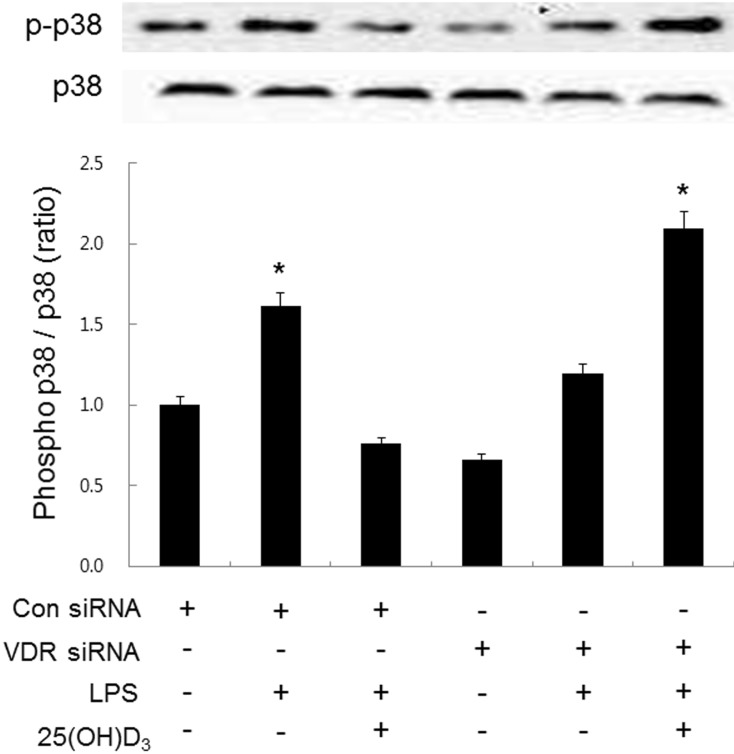
- Microglia are activated by inflammatory and pathophysiological stimuli in neurodegenerative diseases, and activated microglia induce neuronal damage by releasing cytotoxic factors like nitric oxide (NO). Activated microglia synthesize a significant amount of vitamin D3 in the rat brain, and vitamin D3 has an inhibitory effect on activated microglia. To investigate the possible role of vitamin D3 as a negative regulator of activated microglia, we examined the effect of 25-hydroxyvitamin D3 on NO production of lipopolysaccharide (LPS)-stimulated microglia. Treatment with LPS increased the production of NO in primary cultured and BV2 microglial cells. Treatment with 25-hydroxyvitamin D3 inhibited the generation of NO in LPS-activated primary microglia and BV2 cells. In addition to NO production, expression of 1-alpha-hydroxylase and the vitamin D receptor (VDR) was also upregulated in LPS-stimulated primary and BV2 microglia. When BV2 cells were transfected with 1-alpha-hydroxylase siRNA or VDR siRNA, the inhibitory effect of 25-hydroxyvitamin D3 on activated BV2 cells was suppressed. 25-Hydroxyvitamin D3 also inhibited the increased phosphorylation of p38 seen in LPS-activated BV2 cells, and this inhibition was blocked by VDR siRNA. The present study shows that 25-hydroxyvitamin D3 inhibits NO production in LPS-activated microglia through the mediation of LPS-induced 1-alpha-hydroxylase. This study also shows that the inhibitory effect of 25-hydroxyvitamin D3 on NO production might be exerted by inhibiting LPS-induced phosphorylation of p38 through the mediation of VDR signaling. These results suggest that vitamin D3 might have an important role in the negative regulation of microglial activation.
MeSH Terms
Figure
Reference
-
1. Lue LF, Kuo YM, Beach T, Walker DG. Microglia activation and anti-inflammatory regulation in Alzheimer's disease. Mol Neurobiol. 2010; 41:115–128. PMID: 20195797.
Article2. Fang F, Lue LF, Yan S, Xu H, Luddy JS, Chen D, Walker DG, Stern DM, Yan S, Schmidt AM, Chen JX, Yan SS. RAGE-dependent signaling in microglia contributes to neuroinflammation, Abeta accumulation, and impaired learning/memory in a mouse model of Alzheimer's disease. FASEB J. 2010; 24:1043–1055. PMID: 19906677.3. Long-Smith CM, Sullivan AM, Nolan YM. The influence of microglia on the pathogenesis of Parkinson's disease. Prog Neurobiol. 2009; 89:277–287. PMID: 19686799.
Article4. Town T, Nikolic V, Tan J. The microglial "activation" continuum: from innate to adaptive responses. J Neuroinflammation. 2005; 2:24. PMID: 16259628.
Article5. Garden GA, Möller T. Microglia biology in health and disease. J Neuroimmune Pharmacol. 2006; 1:127–137. PMID: 18040779.
Article6. Possel H, Noack H, Putzke J, Wolf G, Sies H. Selective upregulation of inducible nitric oxide synthase (iNOS) by lipopolysaccharide (LPS) and cytokines in microglia: in vitro and in vivo studies. Glia. 2000; 32:51–59. PMID: 10975910.
Article7. Saura J. Microglial cells in astroglial cultures: a cautionary note. J Neuroinflammation. 2007; 4:26. PMID: 17937799.
Article8. Brown GC, Neher JJ. Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol Neurobiol. 2010; 41:242–247. PMID: 20195798.
Article9. Lee JW, Bae CJ, Choi YJ, Kim SI, Kim NH, Lee HJ, Kim SS, Kwon YS, Chun W. 3,4,5-Trihydroxycinnamic Acid Inhibits LPS-Induced iNOS Expression by Suppressing NF-κB Activation in BV2 Microglial Cells. Korean J Physiol Pharmacol. 2012; 16:107–112. PMID: 22563255.
Article10. Norman AW. The history of the discovery of vitamin D and its daughter steroid hormone. Ann Nutr Metab. 2012; 61:199–206. PMID: 23183289.
Article11. Cohen-Lahav M, Douvdevani A, Chaimovitz C, Shany S. The anti-inflammatory activity of 1,25-dihydroxyvitamin D3 in macrophages. J Steroid Biochem Mol Biol. 2007; 103:558–562. PMID: 17267205.12. Fritsche J, Mondal K, Ehrnsperger A, Andreesen R, Kreutz M. Regulation of 25-hydroxyvitamin D3-1 alpha-hydroxylase and production of 1 alpha,25-dihydroxyvitamin D3 by human dendritic cells. Blood. 2003; 102:3314–3316. PMID: 12855575.13. Monkawa T, Yoshida T, Hayashi M, Saruta T. Identification of 25-hydroxyvitamin D3 1alpha-hydroxylase gene expression in macrophages. Kidney Int. 2000; 58:559–568. PMID: 10916079.14. Smolders J, Schuurman KG, van Strien ME, Melief J, Hendrickx D, Hol EM, van Eden C, Luchetti S, Huitinga I. Expression of vitamin D receptor and metabolizing enzymes in multiple sclerosis-affected brain tissue. J Neuropathol Exp Neurol. 2013; 72:91–105. PMID: 23334593.
Article15. Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005; 29:21–30. PMID: 15589699.16. Min B. Effects of vitamin d on blood pressure and endothelial function. Korean J Physiol Pharmacol. 2013; 17:385–392. PMID: 24227938.
Article17. Neveu I, Naveilhan P, Menaa C, Wion D, Brachet P, Garabédian M. Synthesis of 1,25-dihydroxyvitamin D3 by rat brain macrophages in vitro. J Neurosci Res. 1994; 38:214–220. PMID: 8078106.18. Garcion E, Nataf S, Berod A, Darcy F, Brachet P. 1,25-Dihydroxyvitamin D3 inhibits the expression of inducible nitric oxide synthase in rat central nervous system during experimental allergic encephalomyelitis. Brain Res Mol Brain Res. 1997; 45:255–267. PMID: 9149100.
Article19. Garcion E, Sindji L, Montero-Menei C, Andre C, Brachet P, Darcy F. Expression of inducible nitric oxide synthase during rat brain inflammation: regulation by 1,25-dihydroxyvitamin D3. Glia. 1998; 22:282–294. PMID: 9482214.
Article20. Lefebvre d'Hellencourt C, Montero-Menei CN, Bernard R, Couez D. Vitamin D3 inhibits proinflammatory cytokines and nitric oxide production by the EOC13 microglial cell line. J Neurosci Res. 2003; 71:575–582. PMID: 12548714.21. Kim JS, Ryu SY, Yun I, Kim WJ, Lee KS, Park JW, Kim YI. 1alpha,25-Dihydroxyvitamin D(3) Protects Dopaminergic Neurons in Rodent Models of Parkinson's Disease through Inhibition of Microglial Activation. J Clin Neurol. 2006; 2:252–257. PMID: 20396528.22. Wergeland S, Torkildsen Ø, Myhr KM, Aksnes L, Mørk SJ, Bø L. Dietary vitamin D3 supplements reduce demyelination in the cuprizone model. PLoS One. 2011; 6:e26262. PMID: 22028844.
Article23. Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr. 1999; 69:842–856. PMID: 10232622.
Article24. Wolpowitz D, Gilchrest BA. The vitamin D questions: how much do you need and how should you get it? J Am Acad Dermatol. 2006; 54:301–317. PMID: 16443061.
Article25. Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol. 1990; 27:229–237. PMID: 2110186.26. Choi Y, Lee MK, Lim SY, Sung SH, Kim YC. Inhibition of inducible NO synthase, cyclooxygenase-2 and interleukin-1beta by torilin is mediated by mitogen-activated protein kinases in microglial BV2 cells. Br J Pharmacol. 2009; 156:933–940. PMID: 19298258.
Article27. Hu LF, Wong PT, Moore PK, Bian JS. Hydrogen sulfide attenuates lipopolysaccharide-induced inflammation by inhibition of p38 mitogen-activated protein kinase in microglia. J Neurochem. 2007; 100:1121–1128. PMID: 17212697.
Article28. Kim BW, Koppula S, Hong SS, Jeon SB, Kwon JH, Hwang BY, Park EJ, Choi DK. Regulation of microglia activity by glaucocalyxin-A: attenuation of lipopolysaccharide-stimulated neuroinflammation through NF-κB and p38 MAPK signaling pathways. PLoS One. 2013; 8:e55792. PMID: 23393601.
Article29. Sakai A, Takasu K, Sawada M, Suzuki H. Hemokinin-1 gene expression is upregulated in microglia activated by lipopolysaccharide through NF-κB and p38 MAPK signaling pathways. PLoS One. 2012; 7:e32268. PMID: 22384199.
Article30. Uesugi M, Nakajima K, Tohyama Y, Kohsaka S, Kurihara T. Nonparticipation of nuclear factor kappa B (NFkappaB) in the signaling cascade of c-Jun N-terminal kinase (JNK)- and p38 mitogen-activated protein kinase (p38MAPK)-dependent tumor necrosis factor alpha (TNFalpha) induction in lipopolysaccharide (LPS)-stimulated microglia. Brain Res. 2006; 1073-1074:48–59. PMID: 16457791.31. Nakajima K, Tohyama Y, Kohsaka S, Kurihara T. Protein kinase C alpha requirement in the activation of p38 mitogenactivated protein kinase, which is linked to the induction of tumor necrosis factor alpha in lipopolysaccharide-stimulated microglia. Neurochem Int. 2004; 44:205–214. PMID: 14602083.32. Ding C, Wilding JP, Bing C. 1,25-dihydroxyvitamin D3 protects against macrophage-induced activation of NFκB and MAPK signalling and chemokine release in human adipocytes. PLoS One. 2013; 8:e61707. PMID: 23637889.
Article33. Zhang Y, Leung DY, Richers BN, Liu Y, Remigio LK, Riches DW, Goleva E. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol. 2012; 188:2127–2135. PMID: 22301548.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Seongpungtang on the Nitric oxide Production of C(6) glial cell and Microglia
- A combinational effect of acetaminophen and oriental herbs on the regulation of inflammatory mediators in microglia cell line, BV2
- Effects of Taxol on the Synthesis of Nitric Oxide in Murine Microglial Cells
- Effect of Erythropoietin on the Production of Nitric Oxide in Trabecular Meshwork Cells
- The Effects of Topical Carbonic Anhydrase Inhibitors on Nitric Oxide Production in Trabecular Meshwork Cells