Korean J Physiol Pharmacol.
2011 Dec;15(6):327-332. 10.4196/kjpp.2011.15.6.327.
Chronic Opium Treatment Can Differentially Induce Brain and Liver Cells Apoptosis in Diabetic and Non-diabetic Male and Female Rats
- Affiliations
-
- 1Department of Biochemistry, Faculty of Medicine, Qazvin University of Medical Sciences, Qazvin, P.O.Box: 7515412578, Iran.
- 2Department of Biochemistry and Physiology Research Center, Kerman University of Medical Sciences, Kerman, P.O.Box: 7616914115, Iran. gh_asadi@kmu.ac.ir
- 3Department of Pathology, Faculty of Medicine, Rafsanjan University of Medical Sciences, Rafsanjan, P.O.Box: 7719617996, Iran.
- 4Department of Biochemistry, Faculty of Medicine, Rafsanjan University of Medical Sciences, Rafsanjan, P.O.Box: 7719617996, Iran.
- 5Department of Hematology, Faculty of Medicine, Rafsanjan University of Medical Sciences, Rafsanjan, P.O.Box: 7719617996, Iran.
- 6Department of Biochemistry, Faculty of Medicine, Zahedan University of Medical Sciences, Zahedan, P.O.Box: 9816743463, Iran.
- 7Department of Physiology and Physiology Research Center, Kerman University of Medical Sciences, Kerman, P.O.Box: 7616914115, Iran.
- KMID: 2285418
- DOI: http://doi.org/10.4196/kjpp.2011.15.6.327
Abstract
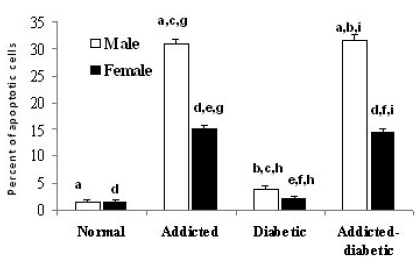
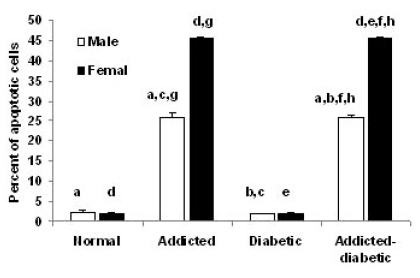
- It has been shown that some opium derivatives promote cell death via apoptosis. This study was designed to examine the influence of opium addiction on brain and liver cells apoptosis in male and female diabetic and non-diabetic Wistar rats. This experimental study was performed on normal, opium-addicted, diabetic and diabetic opium-addicted male and female rats. Apoptosis was evaluated by TUNEL and DNA fragmentation assays. Results of this study showed that apoptosis in opium-addicted and diabetic opium-addicted brain and liver cells were significantly higher than the both normal and diabetic rats. In addition, we found that apoptosis in brain cells of opium-addicted and diabetic opium-addicted male rats were significantly higher than opium-addicted and diabetic opium-addicted female, whereas apoptosis in liver cells of opium-addicted and diabetic opium-addicted female rats were significantly higher than opium-addicted and diabetic opium-addicted male. Overall, these results indicate that opium probably plays an important role in brain and liver cells apoptosis, therefore, leading neurotoxicity and hepatotoxicity. These findings also in away possibly means that male brain cells are more susceptible than female and interestingly liver of females are more sensitive than males in induction of apoptosis by opium.
Keyword
MeSH Terms
Figure
Reference
-
1. Sastry PS, Rao KS. Apoptosis and the nervous system. J Neurochem. 2000; 74:1–20. PMID: 10617101.
Article2. Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001; 410:988–994. PMID: 11309629.
Article3. Yin D, Woodruff M, Zhang Y, Whaley S, Miao J, Ferslew K, Zhao J, Stuart C. Morphine promotes Jurkat cell apoptosis through pro-apoptotic FADD/P53 and anti-apoptotic PI3K/Akt/NF-kappaB pathways. J Neuroimmunol. 2006; 174:101–107. PMID: 16529824.4. Emeterio EP, Tramullas M, Hurlé MA. Modulation of apoptosis in the mouse brain after morphine treatments and morphine withdrawal. J Neurosci Res. 2006; 83:1352–1361. PMID: 16496378.
Article5. Boronat MA, García-Fuster MJ, García-Sevilla JA. Chronic morphine induces up-regulation of the pro-apoptotic Fas receptor and down-regulation of the anti-apoptotic Bcl-2 oncoprotein in rat brain. Br J Pharmacol. 2001; 134:1263–1270. PMID: 11704646.
Article6. Singhal P, Kapasi A, Reddy K, Franki N. Opiates promote T cell apoptosis through JNK and caspase pathway. Adv Exp Med Biol. 2001; 493:127–135. PMID: 11727758.
Article7. Jaume M, Jacquet S, Cavaillès P, Macè G, Stephan L, Blanpied C, Demur C, Brousset P, Dietrich G. Opioid receptor blockade reduces Fas-induced hepatitis in mice. Hepatology. 2004; 40:1136–1143. PMID: 15389866.
Article8. Cunha-Oliveira T, Rego AC, Garrido J, Borges F, Macedo T, Oliveira CR. Street heroin induces mitochondrial dysfunction and apoptosis in rat cortical neurons. J Neurochem. 2007; 101:543–554. PMID: 17250679.
Article9. Hitosugi N, Hatsukari I, Ohno R, Hashimoto K, Mihara S, Mizukami S, Nakamura S, Sakagami H, Nagasaka H, Matsumoto I, Kawase M. Comparative analysis of apoptosisinducing activity of codeine and codeinone. Anesthesiology. 2003; 98:643–650. PMID: 12606908.
Article10. Schiff PL. Opium and its alkaloids. Am J Pharm Educ. 2002; 66:186–194.11. Karam GA, Reisi M, Kaseb AA, Khaksari M, Mohammadi A, Mahmoodi M. Effects of opium addiction on some serum factors in addicts with non-insulin-dependent diabetes mellitus. Addict Biol. 2004; 9:53–58. PMID: 15203439.
Article12. Karam GA, Rashidinejad HR, Aghaee MM, Ahmadi J, Rahmani MR, Mahmoodi M, Azin H, Mirzaee MR, Khaksari M. Opium can differently alter blood glucose, sodium and potassium in male and female rats. Pak J Pharm Sci. 2008; 21:180–184. PMID: 18390449.13. Asadikaram G, Asiabanha M, Sayadi A, Jafarzadeh A, Hassanshahi G. Impact of opium on the serum levels of TGF-β in diabetic, addicted and addicted-diabetic rats. Iran J Immunol. 2010; 7:186–192. PMID: 20876989.14. Ye K, Ke Y, Keshava N, Shanks J, Kapp JA, Tekmal RR, Petros J, Joshi HC. Opium alkaloid noscapine is an antitumor agent that arrests metaphase and induces apoptosis in dividing cells. Proc Natl Acad Sci USA. 1998; 95:1601–1606. PMID: 9465062.
Article15. Mahmoudian M, Mojaverian N. Efffect of noscapine, the antitussive opioid alkaloid, on bradykinin-induced smooth muscle contraction in the isolated ileum of the guinea-pig. Acta Physiol Hung. 2001; 88:231–237. PMID: 12162581.16. Newcomb EW, Lukyanov Y, Smirnova I, Schnee T, Zagzag D. Noscapine induces apoptosis in human glioma cells by an apoptosis-inducing factor-dependent pathway. Anticancer Drugs. 2008; 19:553–563. PMID: 18525314.
Article17. Aneja R, Ghaleb AM, Zhou J, Yang VW, Joshi HC. p53 and p21 determine the sensitivity of noscapine-induced apoptosis in colon cancer cells. Cancer Res. 2007; 67:3862–3870. PMID: 17440101.
Article18. Gao YJ, Stead S, Lee RM. Papaverine induces apoptosis in vascular endothelial and smooth muscle cells. Life Sci. 2002; 70:2675–2685. PMID: 12269394.
Article19. Venturella VS. Gennard AR, editor. Natural Product. The Science and Practice of Pharmacy. 1995. 19th ed. Remington: Mack Publishing Company;p. 400–402.20. Buchbauer G, Nikiforov A, Remberg B. Headspace constituents of opium. Planta Med. 1994; 60:181–183. PMID: 8202569.
Article21. Akbarzadeh A, Norouzian D, Mehrabi MR, Jamshidi Sh, Farhangi A, Allah Verdi A, Mofidian1 SMA, Lame Rad B. Induction of diabetes by streptozocin in rats. Indian J Clin Biochem. 2007; 22:60–64.22. Jafari Anarkooli I, Sankian M, Ahmadpour S, Varasteh AR, Haghir H. Evaluation of Bcl-2 family gene expression and Caspase-3 activity in hippocampus STZ-induced diabetic rats. Exp Diabetes Res. 2008; 2008:638467. PMID: 18923682.
Article23. Chawla S, Korenblik A, Kunnen S. Annual prevalence of drug abuse. 2005. Austria: Wold drug report, United Nations Publication, United Nations Office on Drugs and Crime Vienna;p. 363–366.24. Pu L, Bao GB, Xu NJ, Ma L, Pei G. Hippocampal long-term potentiation is reduced by chronic opiate treatment and can be restored by re-exposure to opiates. J Neurosci. 2002; 22:1914–1921. PMID: 11880521.
Article25. Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev. 2001; 81:299–343. PMID: 11152760.
Article26. Mao J, Sung B, Ji RR, Lim G. Neuronal apoptosis associated with morphine tolerance: evidence for an opioid-induced neurotoxic mechanism. J Neurosci. 2002; 22:7650–7661. PMID: 12196588.
Article27. García-Fuster MJ, Ferrer-Alcón M, Miralles A, García-Sevilla JA. Modulation of Fas receptor proteins and dynamin during opiate addiction and induction of opiate withdrawal in rat brain. Naunyn Schmiedebergs Arch Pharmacol. 2003; 368:421–431. PMID: 14530904.28. Sklair-Tavron L, Shi WX, Lane SB, Harris HW, Bunney BS, Nestler EJ. Chronic morphine induces visible changes in the morphology of mesolimbic dopamine neurons. Proc Natl Acad Sci USA. 1996; 93:11202–11207. PMID: 8855333.
Article29. Robinson TE, Gorny G, Savage VR, Kolb B. Widespread but regionally specific effects of experimenter- versus self-administered morphine on dendritic spines in the nucleus accumbens, hippocampus, and neocortex of adult rats. Synapse. 2002; 46:271–279. PMID: 12373743.
Article30. Hsiao PN, Chang MC, Cheng WF, Chen CA, Lin HW, Hsieh CY, Sun WZ. Morphine induces apoptosis of human endothelial cells through nitric oxide and reactive oxygen species pathways. Toxicology. 2009; 256:83–91. PMID: 19070643.
Article31. Faulkner L, Altmann DM, Ellmerich S, Huhtaniemi I, Stamp G, Sriskandan S. Sexual dimorphism in superantigen shock involves elevated TNF-alpha and TNF-alpha induced hepatic apoptosis. Am J Respir Crit Care Med. 2007; 176:473–482. PMID: 17575097.32. Fuggetta MP, Di Francesco P, Falchetti R, Cottarelli A, Rossi L, Tricarico M, Lanzilli G. Effect of morphine on cell-mediated immune responses of human lymphocytes against allogeneic malignant cells. J Exp Clin Cancer Res. 2005; 24:255–263. PMID: 16110759.33. Lang JT, McCullough LD. Pathways to ischemic neuronal cell death: are sex differences relevant? J Transl Med. 2008; 6:33. PMID: 18573200.
Article34. Vagnerova K, Koerner IP, Hurn PD. Gender and the injured brain. Anesth Analg. 2008; 107:201–214. PMID: 18635489.
Article35. Sueoka E, Sueoka N, Kai Y, Okabe S, Suganuma M, Kanematsu K, Yamamoto T, Fujiki H. Anticancer activity of morphine and its synthetic derivative, KT-90, mediated through apoptosis and inhibition of NF-kappaB activation. Biochem Biophys Res Commun. 1998; 252:566–570. PMID: 9837747.36. Maneckjee R, Minna JD. Opioids induce while nicotine suppresses apoptosis in human lung cancer cells. Cell Growth Differ. 1994; 5:1033–1040. PMID: 7848904.37. Liu M, Dziennis S, Hurn PD, Alkayed NJ. Mechanisms of gender-linked ischemic brain injury. Restor Neurol Neurosci. 2009; 27:163–179. PMID: 19531872.
Article38. Sas K, Robotka H, Toldi J, Vécsei L. Mitochondria, metabolic disturbances, oxidative stress and the kynurenine system, with focus on neurodegenerative disorders. J Neurol Sci. 2007; 257:221–239. PMID: 17462670.
Article39. Sima AA, Zhang W, Li ZG, Kamiya H. The effects of C-peptide on type 1 diabetic polyneuropathies and encephalopathy in the BB/Wor-rat. Exp Diabetes Res. 2008; 2008:230458. PMID: 18437223.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Relationship between Opium Abuse and Severity of Depression in Type 2 Diabetic Patients
- Blockade of Oxidative Stress by Vitamin C Ameliorates Albuminuria and Renal Sclerosis in Experimental Diabetic Rats
- Proliferation of Cultured Vascular Smooth Muscle Cells(VSMCs) Obtained from Aortas of Insulin Dependent Diabetic Rats
- Effect of irradiation on the acinar cells of submandibular gland in streptozotocin-induced diabetic rats
- Effects of Chronic Treatment with a Type 5 Phosphodiesterase Inhibitor on Erectile Function in Diabetic Rats