Yonsei Med J.
2007 Oct;48(5):847-855. 10.3349/ymj.2007.48.5.847.
Blockade of Oxidative Stress by Vitamin C Ameliorates Albuminuria and Renal Sclerosis in Experimental Diabetic Rats
- Affiliations
-
- 1Department of Internal Medicine and Clinical Research Institute, Soonchunhyang University Cheonan Hospital, Cheonan, Korea.
- 2Department of Internal Medicine, Yonsei University Wonju College of Medicine, Wonju; 3Department of Pathology, Yonsei University College of Medicine, Seoul, Korea. cchung@yonsei.ac.kr
- KMID: 1122624
- DOI: http://doi.org/10.3349/ymj.2007.48.5.847
Abstract
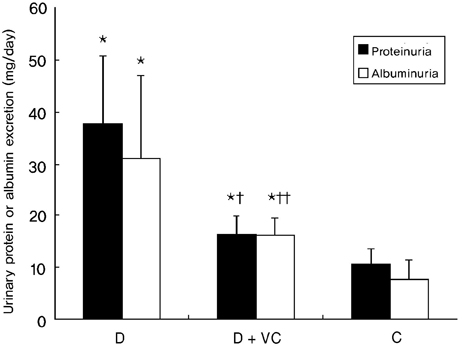
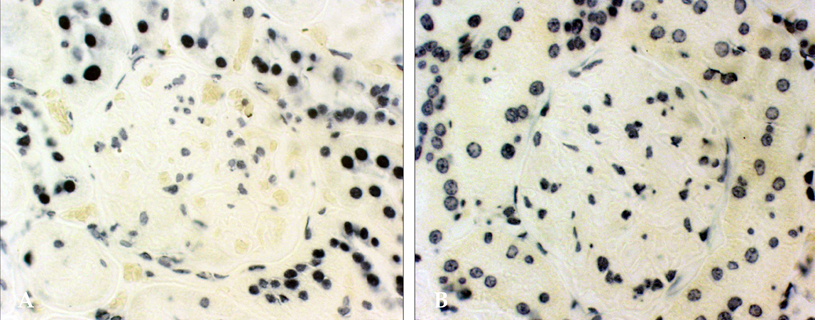
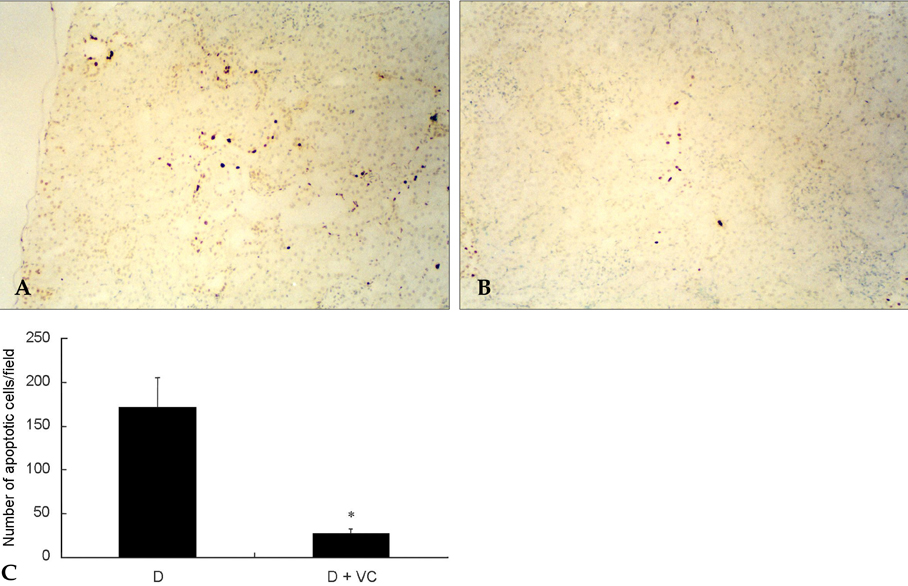
- PURPOSE: Oxidative stress has been suggested to play a role as a common mediator of apoptosis and kidney damage in diabetes. However, it is uncertain whether the apoptosis occurs in the kidney during the course of diabetes. We investigated the occurrence of apoptosis in the diabetic rat kidney, the role of oxidative stress and the effect of an antioxidant on apoptosis in the diabetic rat kidney. MATERIALS AND METHODS: Otsuka-Long-Evans-Tokushima-Fatty rats, an animal model for type 2 diabetes, were randomized into a non-treated diabetic (n=8) and a vitamin C-treated group (n=8). Long-Evans Tokushima Otsuka rats (n=8) were used as a control. RESULTS: Apoptosis was present in the epithelial cells of the proximal tubules in diabetic rats. The number of apoptotic cells, albuminuria, proteinuria, glomerular and tubulointerstitial sclerosis, and renal malondialdehyde were significantly decreased in vitamin C-treated diabetic rats when compared to the untreated diabetic rats. The decreased slit pore density (number of slit pores per underlying glomerular basement membrane length) as assessed by electron microscopy was also significantly restored by treatment with vitamin C without significantly affecting plasma glucose in diabetic rats. CONCLUSION: By blocking these pathophysiologic processes, a blockade of oxidative stress by vitamin C might become a useful adjunct to albuminuria and renal sclerosis in diabetic nephropathy.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Effects of Short Term Antioxidant Cocktail Supplementation on the Oxidative Stress and Inflammatory Response of Renal Inflammation in Diabetic Mice
Seul-Ki Park, Na-Young Park, Yunsook Lim
Korean J Nutr. 2009;42(8):673-681. doi: 10.4163/kjn.2009.42.8.673.
Reference
-
1. Horie K, Miyata T, Maeda K, Miyata S, Sugiyama S, Sakai H, et al. Immunohistochemical colocalization of glycoxidation products and lipid peroxidation products in diabetic renal glomerular lesions. Implication for glycoxidative stress in the pathogenesis of diabetic nephropathy. J Clin Invest. 1997. 100:2995–3004.
Article2. Trachtman H, Futterweit S, Maesaka J, Ma C, Valderrama E, Fuchs A, et al. Taurine ameliorates chronic streptozocin-induced diabetic nephropathy in rats. Am J Physiol. 1995. 269:F429–F438.
Article3. Craven PA, DeRubertis FR, Kagan VE, Melhem M, Studer RK. Effects of supplementation with vitamin C or E on albuminuria, glomerular TGF-beta, and glomerular size in diabetes. J Am Soc Nephrol. 1997. 8:1405–1414.
Article4. Bursell SE, Clermont AC, Aiello LP, Aiello LM, Schlossman DK, Feener EP, et al. High-dose vitamin E supplementation normalizes retinal blood flow and creatinine clearance in patients with type 1 diabetes. Diabetes Care. 1999. 22:1245–1251.
Article5. Buttke TM, Sandstrom PA. Oxidative stress as a mediator of apoptosis. Immunol Today. 1994. 15:7–10.
Article6. Jee SH, Kim HJ, Lee J. Obesity insulin resistance and cancer risk. Yonsei Med J. 2005. 46:449–455.
Article7. Allen DA, Harwood S, Varagunam M, Raftery MJ, Yaqoob MM. High glucose-induced oxidative stress causes apoptosis in proximal tubular epithelial cells and is mediated by multiple caspases. FASEB J. 2003. 17:908–910.
Article8. Chen L, Jia RH, Qiu CJ, Ding G. Hyperglycemia inhibits the uptake of dehydroascorbate in tubular epithelial cell. Am J Nephrol. 2005. 25:459–465.
Article9. Serbecic N, Beutelspacher SC. Vitamins inhibit oxidant-induced apoptosis of corneal endothelial cells. Jpn J Ophthalmol. 2005. 49:355–362.
Article10. Kang SA, Jang YJ, Park H. In vivo dual effects of vitamin C on paraquat-induced lung damage: dependence on released metals from the damaged tissue. Free Radic Res. 1998. 28:93–107.11. Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes. 1992. 41:1422–1428.
Article12. Bradley M, Schumann GB, Ward PCJ. Henry JB, editor. Examination of urine. Clinical Diagnosis and Management by Laboratory Methods. 1979. Philadelphia: WB Saunders;559–634.13. Rasanayagam LJ, Lim KL, Beng CG, Lau KS. Measurement of urine albumin using bromocresol green. Clin Chim Acta. 1973. 44:53–57.
Article14. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979. 95:351–358.
Article15. Lane PH, Steffes MW, Mauer SM. Estimation of glomerular volume: a comparison of four methods. Kidney Int. 1992. 141:1085–1089.
Article16. Fukuzawa Y, Watanabe Y, Inaguma D, Hotta N. Evaluation of glomerular lesion and abnormal urinary findings in OLETF rats resulting from a long-term diabetic state. J Lab Clin Med. 1996. 128:568–578.
Article17. Thomas SE, Andoh TF, Pichler RH, Shankland SJ, Couser WG, Bennett WM, et al. Accelerated apoptosis characterizes cyclosporine-associated interstitial fibrosis. Kidney Int. 1998. 53:897–908.
Article18. Lahdenkari AT, Lounatmaa K, Patrakka J, Holmberg C, Wartiovaara J, Kestilä M, et al. Podocytes are firmly attached to glomerular basement membrane in kidneys with heavy proteinuria. J Am Soc Nephrol. 2004. 15:2611–2618.
Article19. Iino K, Iwase M, Sonoki K, Yoshinari M, Iida M. Combination treatment of vitamin C and desferrioxamine suppresses glomerular superoxide and prostaglandin E production in diabetic rats. Diabetes Obes Metab. 2005. 7:106–109.
Article20. Kedziora-Kornatowska K, Szram S, Kornatowski T, Szadujkis-Szadurski L, Kedziora J, Bartosz G. Effect of vitamin E and vitamin C supplementation on antioxidative state and renal glomerular basement membrane thickness in diabetic kidney. Nephron Exp Nephrol. 2003. 95:e134–e143.
Article21. Verzola D, Bertolotto MB, Villaggio B, Ottonello L, Dallegri F, Frumento G, et al. Taurine prevents apoptosis induced by high ambient glucose in human tubule renal cells. J Investig Med. 2002. 50:443–451.
Article22. Ishii N, Ogawa Z, Suzuki K, Numakami K, Saruta T, Itoh H. Glucose loading induces DNA fragmentation in rat proximal tubular cells. Metabolism. 1996. 45:1348–1353.
Article23. Ortiz A, Ziyadeh FN, Neilson EG. Expression of apoptosis-regulatory genes in renal proximal tubular epithelial cells exposed to high ambient glucose and in diabetic kidneys. J Investig Med. 1997. 45:50–56.24. Kumar D, Robertson S, Burns KD. Evidence of apoptosis in human diabetic kidney. Mol Cell Biochem. 2004. 259:67–70.
Article25. Zhang W, Khanna P, Chan LL, Campbell G, Ansari NH. Diabetes-induced apoptosis in rat kidney. Biochem Mol Med. 1997. 61:58–62.
Article26. Murata I, Takemura G, Asano K, Sano H, Fujisawa K, Kagawa T, et al. Apoptotic cell loss following cell proliferation in renal glomeruli of Otsuka Long-Evans Tokushima Fatty rats, a model of human type 2 diabetes. Am J Nephrol. 2002. 22:587–595.
Article27. Hirsch IB, Atchley DH, Tsai E, Labbé RF, Chait A. Ascorbic acid clearance in diabetic nephropathy. J Diabetes Complications. 1998. 12:259–263.
Article28. Lal MA, Körner A, Matsuo Y, Zelenin S, Cheng SX, Jaremko G, et al. Combined antioxidant and COMT inhibitor treatment reverses renal abnormalities in diabetic rats. Diabetes. 2000. 49:1381–1389.
Article29. Melhem MF, Craven PA, Derubertis FR. Effects of dietary supplementation of alpha-lipoic acid on early glomerular injury in diabetes mellitus. J Am Soc Nephrol. 2001. 12:124–133.
Article30. Cao G, Booth SL, Sadowski JA, Prior RL. Increases in human plasma antioxidant capacity after consumption of controlled diets high in fruit and vegetables. Am J Clin Nutr. 1998. 68:1081–1087.
Article31. Stackhouse S, Miller PL, Park SK, Meyer TW. Reversal of glomerular hyperfiltration and renal hypertrophy by blood glucose normalization in diabetic rats. Diabetes. 1990. 39:989–995.
Article32. Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, et al. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest. 1997. 99:342–348.
Article33. Kaneto H, Kajimoto Y, Miyagawa J, Matsuoka T, Fujitani Y, Umayahara Y, et al. Beneficial effects of antioxidants in diabetes: possible protection of pancreatic beta-cells against glucose toxicity. Diabetes. 1999. 48:2398–2406.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Changes of Malondialdehyde (MDA) and Antioxidant Enzymes in Patients with Diabetic Nephropathy
- Effects of Vitamin E Supplementation on Oxidative Stress in Streptozotocin Induced Diabetic Rats: Investigation of Liver and Plasma
- Protective Effects of Curcumin on Renal Oxidative Stress and Lipid Metabolism in a Rat Model of Type 2 Diabetic Nephropathy
- The role of oxidative stress and hypoxia in renal disease
- Effects of S-allylcysteine on Oxidative Stress in Streptozotocin-Induced Diabetic Rats