Korean J Physiol Pharmacol.
2008 Dec;12(6):287-291. 10.4196/kjpp.2008.12.6.287.
Pre-ischemic Treatment with Ampicillin Reduces Neuronal Damage in the Mouse Hippocampus and Neostriatum after Transient Forebrain Ischemia
- Affiliations
-
- 1Department of Pharmacology, Cell Death Disease Research Center, College of Medicine, The Catholic University of Korea, Seoul 137-701, Korea. syk@catholic.ac.kr
- KMID: 2285354
- DOI: http://doi.org/10.4196/kjpp.2008.12.6.287
Abstract
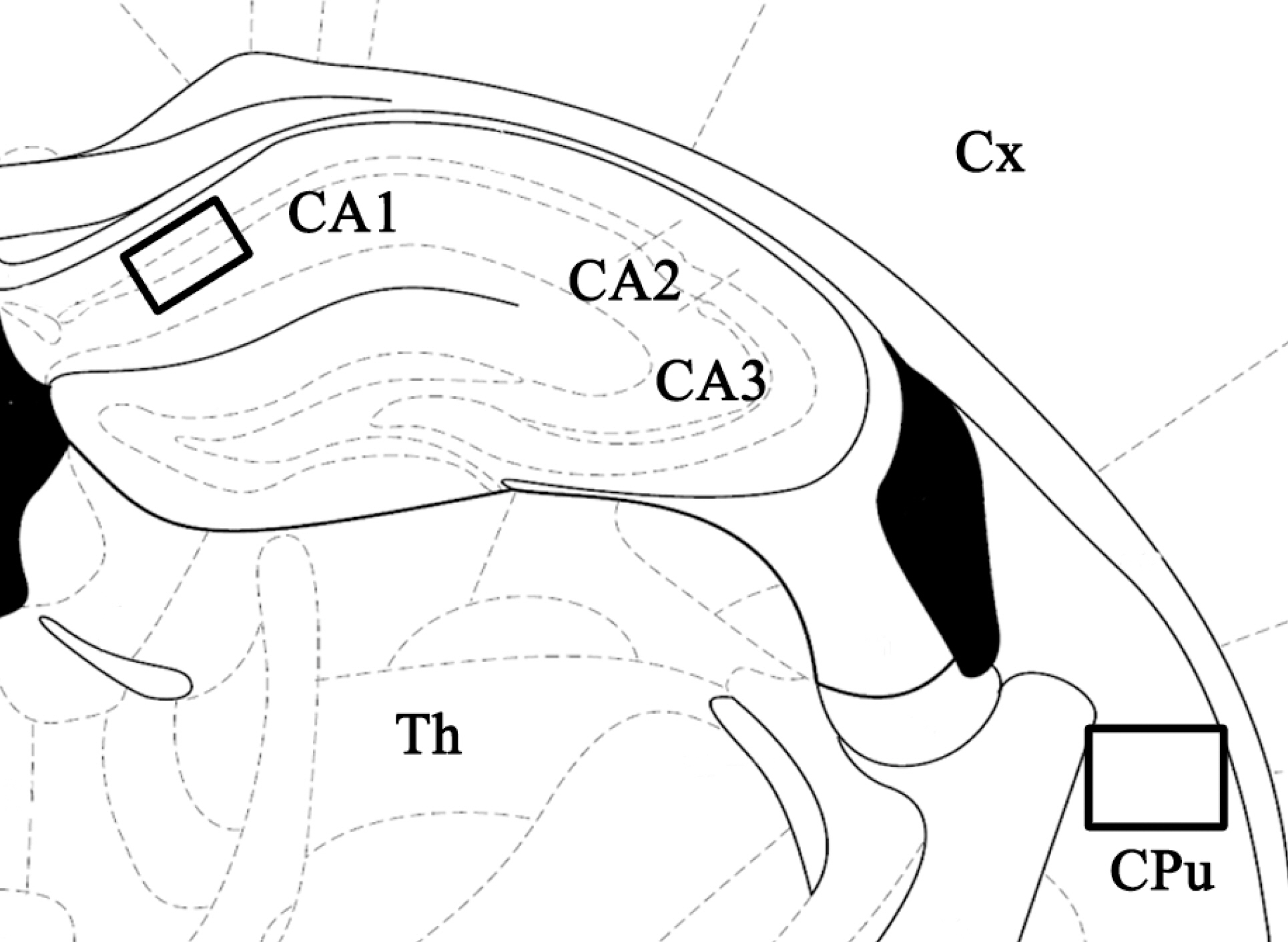
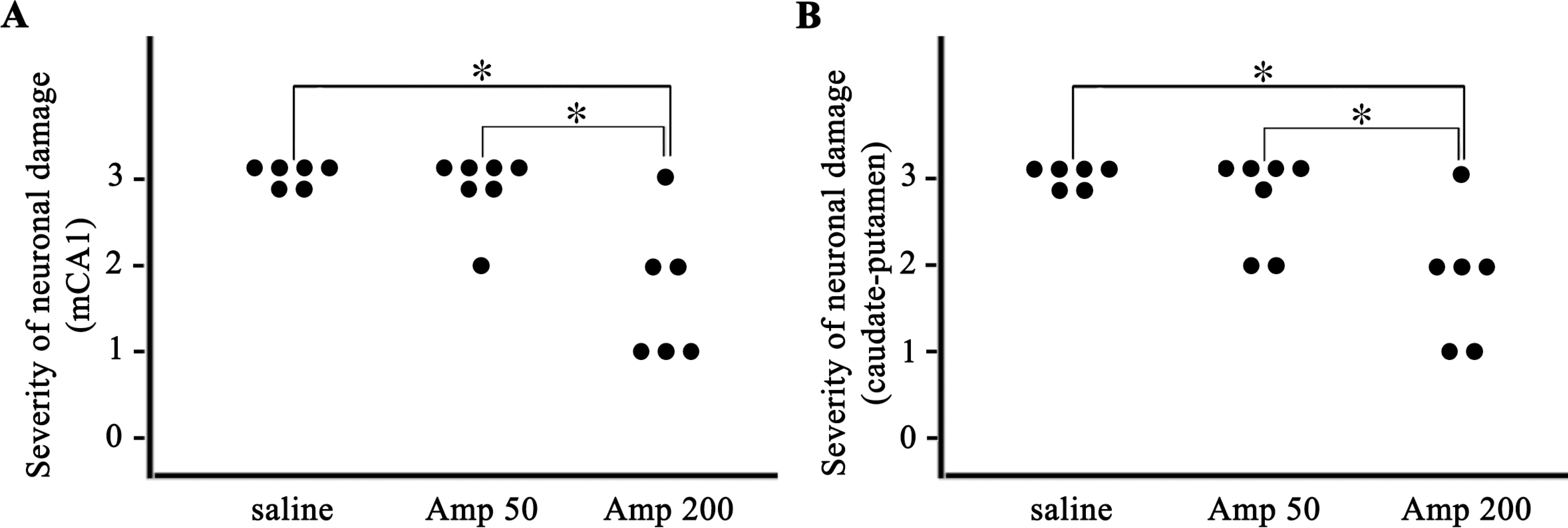
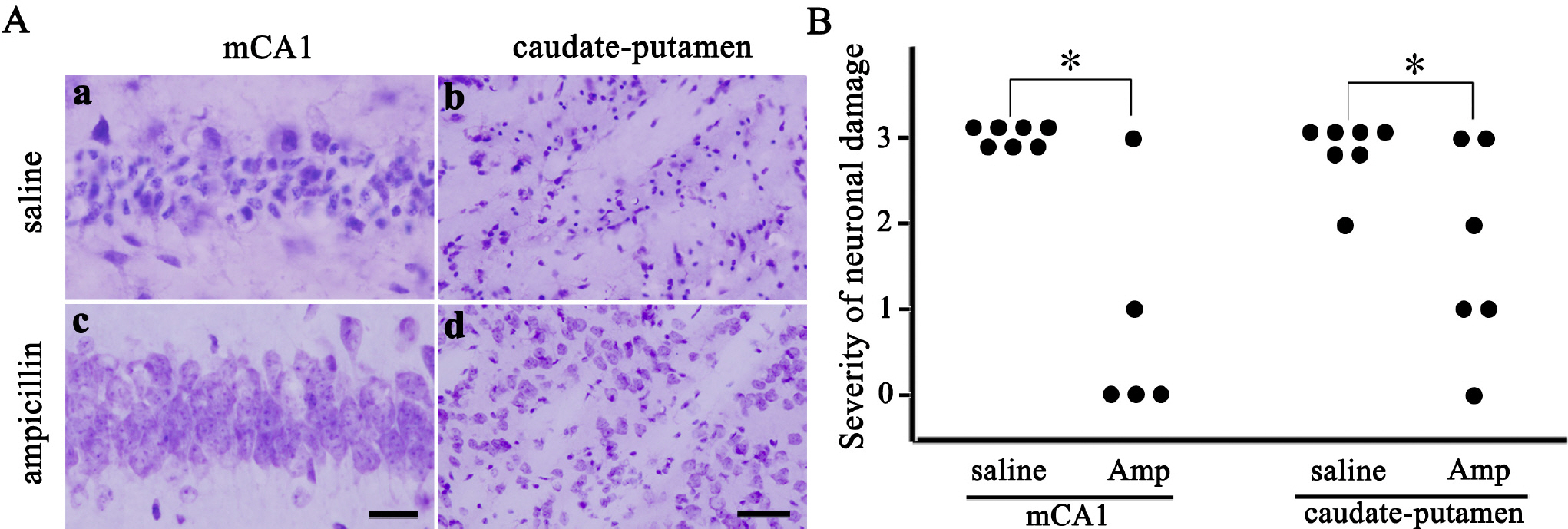
- Ampicillin, a beta-lactam antibiotic, has been reported to induce astrocytic glutamate transporter-1 which plays a crucial role in protecting neurons against glutamate excitotoxicity. We investigated the effect of ampicillin on neuronal damage in the mouse hippocampus and neostriatum following transient global forebrain ischemia. Male C57BL/6 mice were anesthetized with halothane and subjected to bilateral occlusion of the common carotid artery for 40 min. Ampicillin was administered post-ischemically (for 3 days) and/or pre-ischemically (for 3~5 days until one day before the onset of ischemia). Pre- and post-ischemic treatment with ampicillin (50 mg/kg/day or 200 mg/kg/day) prevented ischemic neuronal death in the medial CA1 area of the hippocampus as well as the neostriatum in a dose-dependent manner. In addition, ischemic neuronal damage was reduced by pre-ischemic treatment with ampicillin (200 mg/kg/day). In summary, our results suggest that ampicillin plays a functional role as a chemical preconditioning agent that protects hippocampal neurons from ischemic insult.
MeSH Terms
Figure
Cited by 2 articles
-
The Neuroprotective Potential of Cyanidin-3-glucoside Fraction Extracted from Mulberry Following Oxygen-glucose Deprivation
Mohammad Iqbal Hossain Bhuiyan, Hyun-Bok Kim, Seong Yun Kim, Kyung-Ok Cho
Korean J Physiol Pharmacol. 2011;15(6):353-361. doi: 10.4196/kjpp.2011.15.6.353.The neuroprotective mechanism of ampicillin in a mouse model of transient forebrain ischemia
Kyung-Eon Lee, Kyung-Ok Cho, Yun-Sik Choi, Seong Yun Kim
Korean J Physiol Pharmacol. 2016;20(2):185-192. doi: 10.4196/kjpp.2016.20.2.185.
Reference
-
Anderson CM., Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 32:1–14. 2000.
ArticleBenveniste H. The excitotoxin hypothesis in relation to cerebral ischemia. Cerebrovasc Brain Metab Rev. 3:213–245. 1991.Cho KO., Kim SK., Cho YJ., Sung KW., Kim SY. A simple method for predicting hippocampal neurodegeneration in a mouse model of transient global forebrain ischemia. Korean J Physiol Pharmacol. 10:167–172. 2006.Chu K., Lee ST., Sinn DI., Ko SY., Kim EH., Kim JM., Kim SJ., Park DK., Jung KH., Song EC., Lee SK., Kim M., Roh JK. Pharmacological Induction of Ischemic Tolerance by Glutamate Transporter-1 (EAAT2) Upregulation. Stroke. 38:177–182. 2007.
ArticleEnglish AR., Girard D., Haskell SL. Pharmacokinetics of sultamicillin in mice, rats, and dogs. Antimicrob Agents Chemother. 25:599–602. 1984.
ArticleHuber R., Kasischke K., Ludolph AC., Riepe MW. Increase of cellular hypoxic tolerance by erythromycin and other antibiotics. Neuroreport. 10:1543–1546. 1999.
ArticleJi HF., Shen L., Zhang HY. Beta-lactam antibiotics are multipotent agents to combat neurological diseases. Biochem Biophys Res Commun. 333:661–663. 2005.Kanra G. Experience with ampicillin/sulbactam in severe infections. J Int Med Res. 30(1 Suppl):20A–30A. 2002.
ArticleKirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 239:57–69. 1982.
ArticleLee SG., Su ZZ., Emdad L., Gupta P., Sarkar D., Borjabad A., Volsky DJ., Fisher PB. Mechanism of ceftriaxone induction of excitatory amino acid transporter-2 expression and glutamate uptake in primary human astrocytes. J Biol Chem. 283:13116–13123. 2008.
ArticleLipski J., Wan CK., Bai JZ., Pi R., Li D., Donnelly D. Neuroprotective potential of ceftriaxone in in vitro models of stroke. Neuroscience. 146:617–629. 2007.
ArticleLipton P. Ischemic cell death in brain neurons. Physiol Rev. 79:1431–1568. 1999.
ArticleLo EH., Moskowitz MA., Jacobs TP. Exciting, radical, suicidal: how brain cells die after stroke. Stroke. 36:189–192. 2005.Lutsar I., McCracken GH., Friedland IR. Antibiotic pharmacodynamics in cerebrospinal fluid. Clin Infect Dis. 27:1117–1128. 1998.
ArticleMordenti J., Chappell W. The use of interspecies scaling in toxicokinetics. Yacobi A, Kelly J, Batra V, editors. eds,. Toxicokinetics and new drug development. Pergamon Press;New York: p. p. 42–96. 1989.Murakami K., Kondo T., Kawase M., Chan PH. The development of a new mouse model of global ischemia: focus on the relationships between ischemia duration, anesthesia, cerebral vasculature, and neuronal injury following global ischemia in mice. Brain Res. 780:304–310. 1998.
ArticleOuyang YB., Voloboueva LA., Xu LJ., Giffard RG. Selective dysfunction of hippocampal CA1 astrocytes contributes to delayed neuronal damage after transient forebrain ischemia. J Neurosci. 27:4253–4260. 2007.
ArticlePaxinos G., Franklin KBJ. The mouse brain in stereotaxic coordinates. 2nd ed.Academic Press;London: 2001.Quagliarello VJ., Scheld WM. Treatment of bacterial meningitis. N Engl J Med. 336:708–716. 1997.
ArticleRao VL., Dogan A., Todd KG., Bowen KK., Kim BT., Rothstein JD., Dempsey RJ. Antisense knockdown of the glial glutamate transporter GLT-1, but not the neuronal glutamate transporter EAAC1, exacerbates transient focal cerebral ischemia-induced neuronal damage in rat brain. J Neurosci. 21:1876–1883. 2001.
ArticleRothstein JD., Patel S., Regan MR., Haenggeli C., Huang YH., Bergles DE., Jin L., Dykes Hoberg M., Vidensky S., Chung DS., Toan SV., Bruijn LI., Su ZZ., Gupta P., Fisher PB. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 433:73–77. 2005.Swanson RA., Ying W., Kauppinen TM. Astrocyte influences on ischemic neuronal death. Curr Mol Med. 4:193–205. 2004.
ArticleWatase K., Hashimoto K., Kano M., Yamada K., Watanabe M., Inoue Y., Okuyama S., Sakagawa T., Ogawa S., Kawashima N., Hori S., Takimoto M., Wada K., Tanaka K. Motor discoordination and increased susceptibility to cerebellar injury in GLAST mutant mice. Eur J Neurosci. 10:976–988. 1998.
ArticleZhou J., Sutherland ML. Glutamate transporter cluster formation in astrocytic processes regulates glutamate uptake activity. J Neurosci. 24:6301–6306. 2004.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Corrigendum to: Pre-ischemic treatment with ampicillin reduces neuronal damage in the mouse hippocampus and neostriatum after transient forebrain ischemia
- The neuroprotective mechanism of ampicillin in a mouse model of transient forebrain ischemia
- Behamioral Change and Memory Inpairment Following Transient Forebrain Ischemia in Rats
- A Simple Method for Predicting Hippocampal Neurodegeneration in a Mouse Model of Transient Global Forebrain Ischemia
- Postischemic Treatment with Aminoguanidine Inhibits Peroxynitrite Production in the Rat Hippocampus Following Transient Forebrain Ischemia