Clin Exp Otorhinolaryngol.
2014 Dec;7(4):295-301. 10.3342/ceo.2014.7.4.295.
Role of Caffeic Acid on Collagen Production in Nasal Polyp-Derived Fibroblasts
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Korea University College of Medicine, Seoul, Korea. lhman@korea.ac.kr
- 2Department of Medical Science, Korea University Graduate School, Seoul, Korea.
- 3Institute for Medical Devices Clinical Trial Center, Korea University College of Medicine, Seoul, Korea.
- KMID: 2278370
- DOI: http://doi.org/10.3342/ceo.2014.7.4.295
Abstract
OBJECTIVES
Caffeic acids are known to have anti-oxidant, anti-inflammatory, immunomodulatory, and tissue reparative effects. The purposes of this study were to determine the effect of caffeic acid on transforming growth factor (TGF) beta1-induced myofibroblast differentiation and collagen production, and to determine whether caffeic acid is involved in the antioxidant effect in nasal polyp-derived fibroblasts (NPDFs).
METHODS
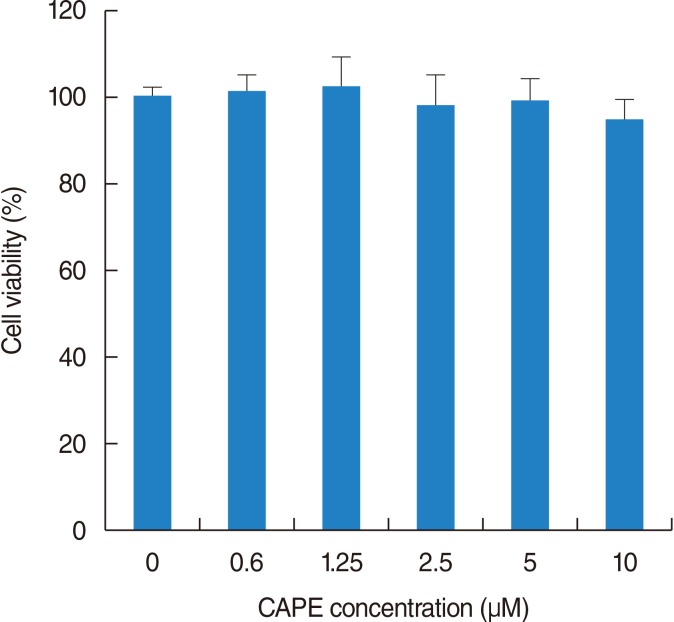
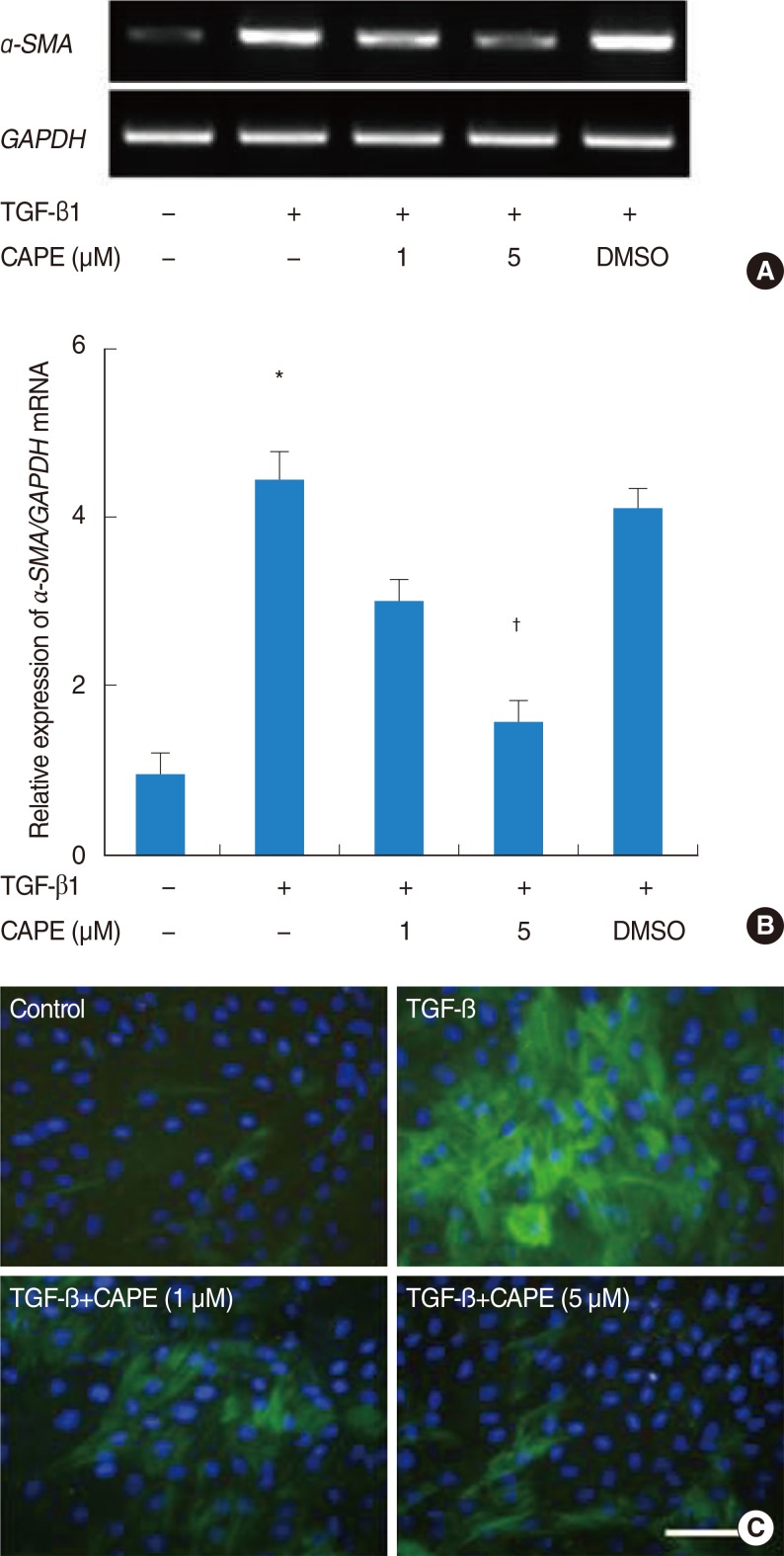
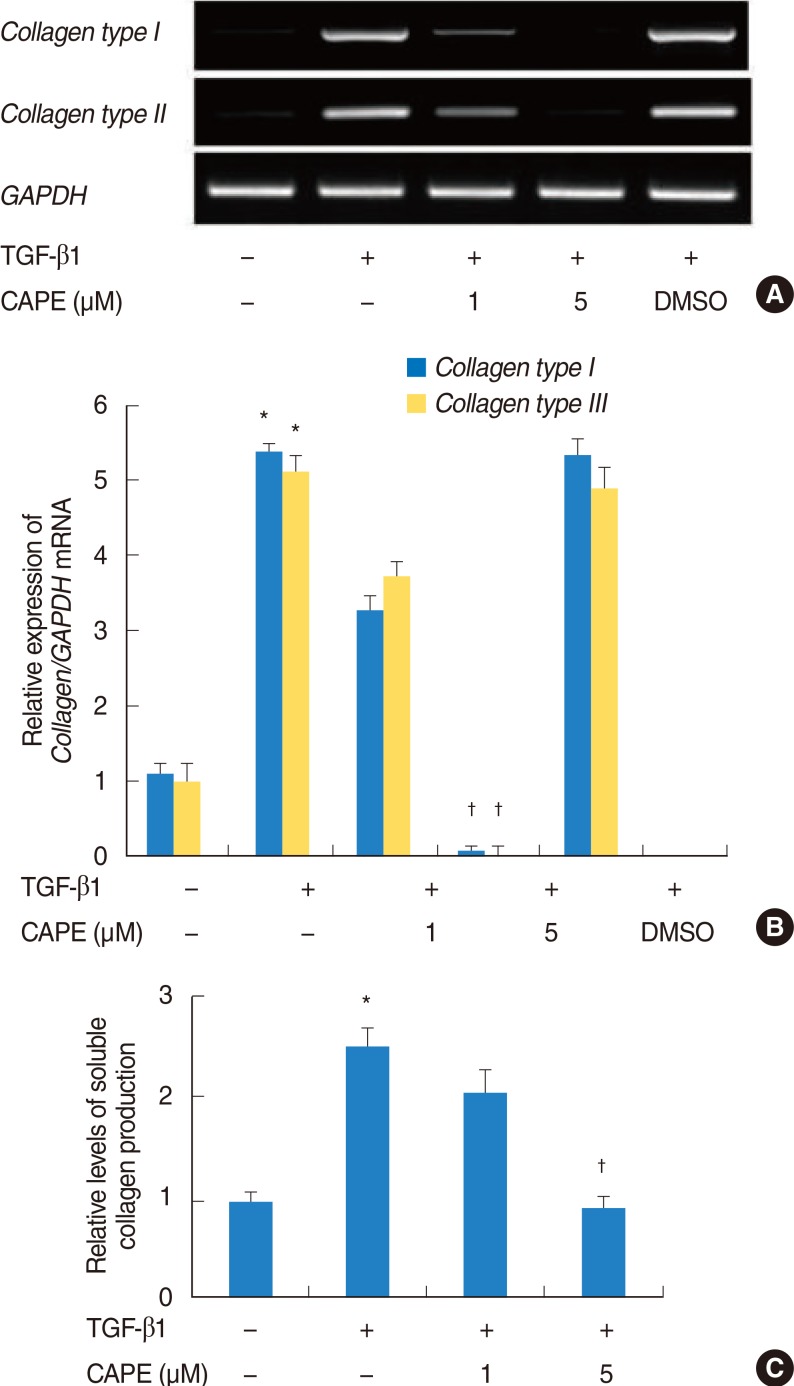
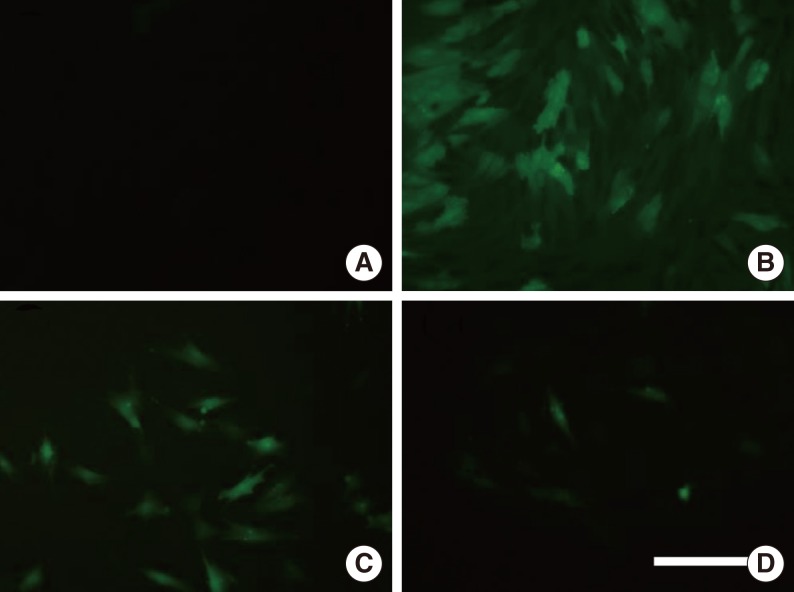
NPDFs were pretreated with caffeic acid (1-10 microM) for 2 hours and stimulated with TGF-beta1 (5 ng/mL) for 24 hours. The expression of alpha-smooth muscle actin (SMA), collagen types I and III, and Nox4 mRNA was determined by a reverse transcription-polymerase chain reaction, and the expression of alpha-SMA protein was determined by actin ned by immunofluorescence microscopy. The amount of total soluble collagen production was analyzed by the Sircol collagen dye-binding assay. The reactive oxygen species (ROS) generated by NPDFs were determined using 2',7'-dichlorfluorescein-diacetate. siNox4 was used to determine the effect of Nox4.
RESULTS
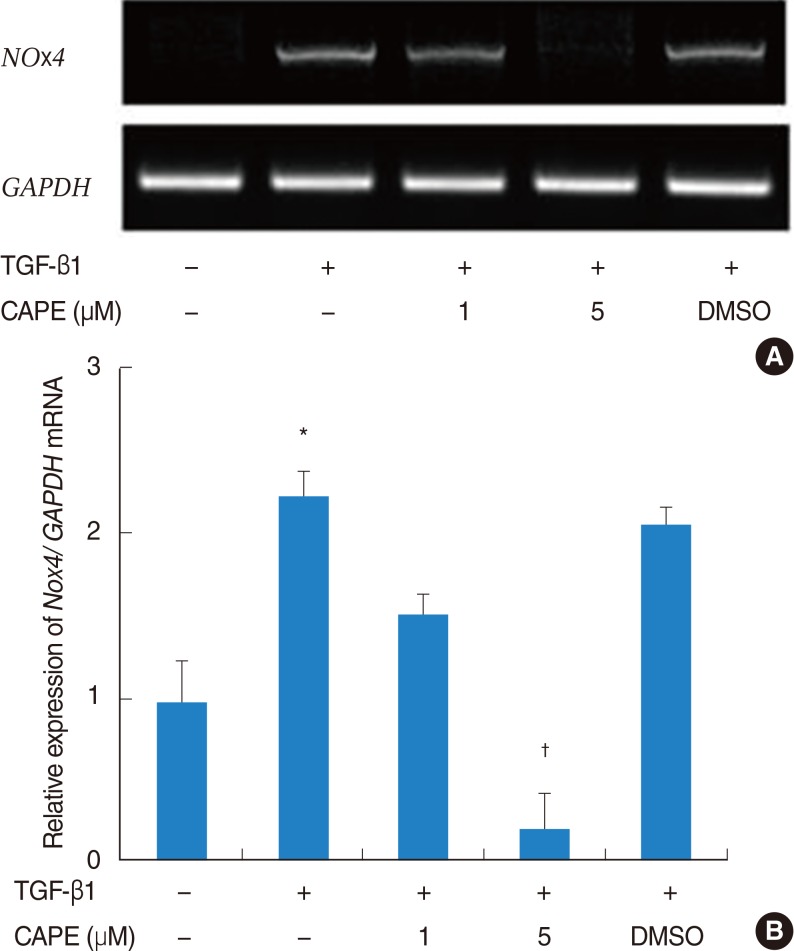
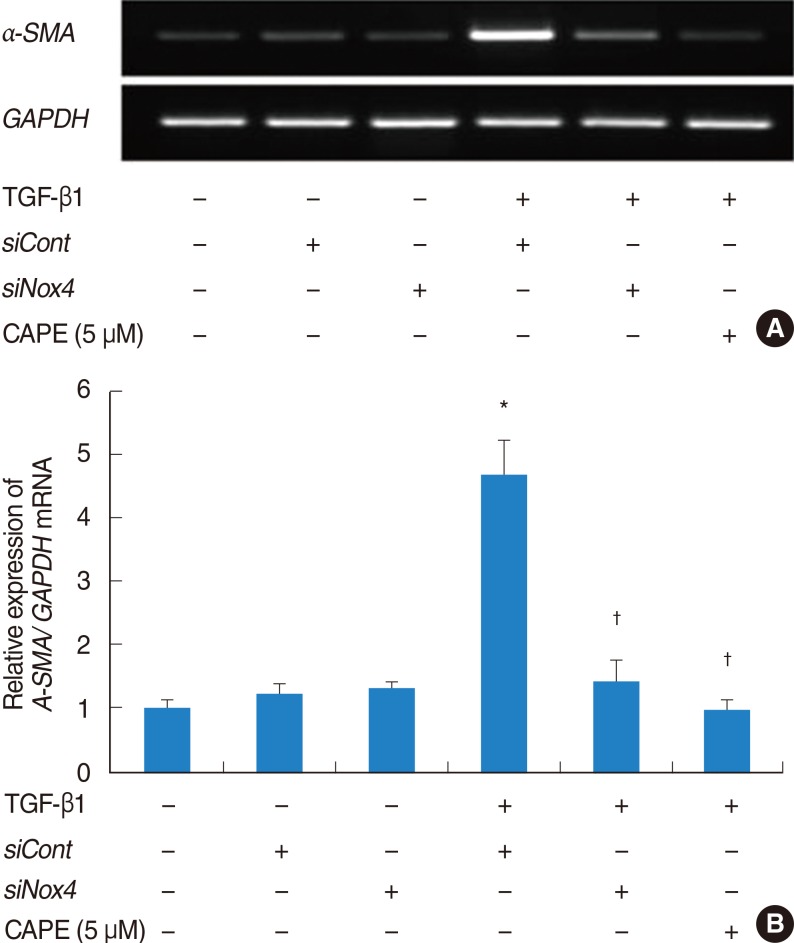
The expression of alpha-SMA and production of collagen were significantly increased following TGF-beta1 treatment. In contrast, the level of expression of alpha-SMA and the level of production of collagen were decreased by pretreatment with caffeic acid. The activation of Nox4 and the subsequent production of ROS were also reduced by pretreatment with caffeic acid. The expression of alpha-SMA was prevented by inhibition of ROS generation with siNox4.
CONCLUSION
Caffeic acid may inhibit TGF-beta1-induced differentiation of fibroblasts into myofibroblasts and collagen production by regulating ROS.
Keyword
MeSH Terms
-
Actins
Antioxidants
Caffeic Acids
Collagen*
Fibroblasts*
Microscopy, Fluorescence
Myofibroblasts
Nasal Polyps
Reactive Oxygen Species
RNA, Messenger
Transforming Growth Factor beta1
Transforming Growth Factors
Actins
Antioxidants
Caffeic Acids
Collagen
RNA, Messenger
Reactive Oxygen Species
Transforming Growth Factor beta1
Transforming Growth Factors
Figure
Reference
-
1. Pawankar R. Nasal polyposis: an update: editorial review. Curr Opin Allergy Clin Immunol. 2003; 2. 3(1):1–6. PMID: 12582307.2. Pawliczak R, Lewandowska-Polak A, Kowalski ML. Pathogenesis of nasal polyps: an update. Curr Allergy Asthma Rep. 2005; 11. 5(6):463–471. PMID: 16216171.
Article3. Wang QP, Escudier E, Roudot-Thoraval F, Abd-Al Samad I, Peynegre R, Coste A. Myofibroblast accumulation induced by transforming growth factor-beta is involved in the pathogenesis of nasal polyps. Laryngoscope. 1997; 7. 107(7):926–931. PMID: 9217133.4. Levi-Schaffer F, Garbuzenko E, Rubin A, Reich R, Pickholz D, Gillery P, et al. Human eosinophils regulate human lung- and skin-derived fibroblast properties in vitro: a role for transforming growth factor beta (TGF-beta). Proc Natl Acad Sci U S A. 1999; 8. 96(17):9660–9665. PMID: 10449750.5. Bradley DT, Kountakis SE. Role of interleukins and transforming growth factor-beta in chronic rhinosinusitis and nasal polyposis. Laryngoscope. 2005; 4. 115(4):684–686. PMID: 15805881.6. Little SC, Early SB, Woodard CR, Shonka DC Jr, Han JK, Borish L, et al. Dual action of TGF-beta1 on nasal-polyp derived fibroblasts. Laryngoscope. 2008; 2. 118(2):320–324. PMID: 18090870.7. Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000; 11. 408(6809):239–247. PMID: 11089981.
Article8. Nagata M. Inflammatory cells and oxygen radicals. Curr Drug Targets Inflamm Allergy. 2005; 8. 4(4):503–504. PMID: 16101529.
Article10. Foley S, Navaratnam S, McGarvey DJ, Land EJ, Truscott TG, Rice-Evans CA. Singlet oxygen quenching and the redox properties of hydroxycinnamic acids. Free Radic Biol Med. 1999; 5. 26(9-10):1202–1208. PMID: 10381191.
Article11. Kikuzaki H, Hisamoto M, Hirose K, Akiyama K, Taniguchi H. Antioxidant properties of ferulic acid and its related compounds. J Agric Food Chem. 2002; 3. 50(7):2161–2168. PMID: 11902973.
Article12. Meyer AS, Frankel EN. Antioxidant activity of hydroxycinnamic acids on human low-density lipoprotein oxidation. Methods Enzymol. 2001; 335:256–265. PMID: 11400373.
Article13. Park IH, Park SJ, Cho JS, Moon YM, Kim TH, Lee SH, et al. Role of reactive oxygen species in transforming growth factor beta1-induced alpha smooth-muscle actin and collagen production in nasal polyp-derived fibroblasts. Int Arch Allergy Immunol. 2012; 10. 159(3):278–286. PMID: 22722757.
Article14. Marquez N, Sancho R, Macho A, Calzado MA, Fiebich BL, Munoz E. Caffeic acid phenethyl ester inhibits T-cell activation by targeting both nuclear factor of activated T-cells and NF-kappaB transcription factors. J Pharmacol Exp Ther. 2004; 3. 308(3):993–1001. PMID: 14617683.15. Liao HF, Chen YY, Liu JJ, Hsu ML, Shieh HJ, Liao HJ, et al. Inhibitory effect of caffeic acid phenethyl ester on angiogenesis, tumor invasion, and metastasis. J Agric Food Chem. 2003; 12. 51(27):7907–7912. PMID: 14690372.
Article16. Park HH, Park IH, Cho JS, Lee YM, Lee HM. The effect of macrolides on myofibroblast differentiation and collagen production in nasal polyp-derived fibroblasts. Am J Rhinol Allergy. 2010; Sep-Oct. 24(5):348–353. PMID: 21244734.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Manuka Honey on Transforming Growth Factor Beta-1-Induced Extracelluar Matrix Production in Nasal Polyp Derived Fibroblasts
- Local Production of IgE in Nasal Polyp
- The Effect of Caffeic Acid on Wound Healing in Skin-incised Mice
- Effects of Caffeic Acid, Myristicin and Rosemarinic Acid on the Gene Expression and Production of Airway MUC5AC Mucin
- Alternaria Induces Production of Thymic Stromal Lymphopoietin in Nasal Fibroblasts Through Toll-like Receptor 2